Efficacy of vildagliptin for prevention of postpartum diabetes in women with a recent history of insulin-requiring gestational diabetes: A phase II, randomized, double-blind, placebo-controlled study
- PMID: 29396374
- PMCID: PMC5869734
- DOI: 10.1016/j.molmet.2017.12.015
Efficacy of vildagliptin for prevention of postpartum diabetes in women with a recent history of insulin-requiring gestational diabetes: A phase II, randomized, double-blind, placebo-controlled study
Abstract
Objective: Women with insulin-requiring gestational diabetes mellitus (GDM) are at high risk of developing diabetes within a few years postpartum. We implemented this phase II study to test the hypothesis that vildagliptin, a dipeptidyl peptidase-4 inhibitor, is superior to placebo in terms of reducing the risk of postpartum diabetes.
Methods: Women with insulin-requiring GDM were randomized to either placebo or 50 mg vildagliptin twice daily for 24 months followed by a 12-month observation period (EudraCT: 2007-000634-39). Both groups received lifestyle counseling. The primary efficacy outcomes were the diagnosis of diabetes (American Diabetes Association (ADA) criteria) or impaired fasting glucose (IFG)/impaired glucose tolerance (IGT).
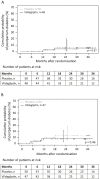
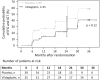
Results: Between 2008 and 2015, 113 patients (58 vildagliptin, 55 placebo) were randomized within 2.2-10.4 (median 8.6) months after delivery. At the interim analysis, nine diabetic events and 28 IFG/IGT events had occurred. Fifty-two women withdrew before completing the treatment phase. Because of the low diabetes rate, the study was terminated. Lifestyle adherence was similar in both groups. At 24 months, the cumulative probability of postpartum diabetes was 3% and 5% (hazard ratio: 1.03; 95% confidence interval: 0.15-7.36) and IFG/IGT was 43% and 22% (hazard ratio: 0.55; 95% confidence interval: 0.26-1.19) in the placebo and vildagliptin groups, respectively. Vildagliptin was well tolerated with no unexpected adverse events.
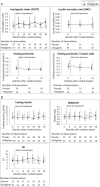
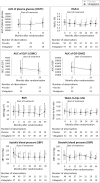
Conclusions: The study did not show significant superiority of vildagliptin over placebo in terms of reducing the risk of postpartum diabetes. However, treatment was safe and suggested some improvements in glycemic control, insulin resistance, and β-cell function. The study identified critical issues in performing clinical trials in the early postpartum period in women with GDM hampering efficacy assessments. With this knowledge, we have set a basis for which properly powered trials could be performed in women with recent GDM. TRIAL REGISTRATION NUMBER AT CLINICALTRIALS.GOV: NCT01018602.
Keywords: Dipeptidyl peptidase-4 inhibitor; Gestational diabetes mellitus; Life-style; Postpartum diabetes; Prevention; Randomized controlled trial.
Copyright © 2018 The Authors. Published by Elsevier GmbH.. All rights reserved.
Figures
References
-
- International Diabetes Federation . 7th ed. International Diabetes Federation; Brussels, Belgium: 2015. IDF diabetes atlas.http://www.diabetesatlas.org
-
- Damm P., Houshmand-Oeregaard A., Kelstrup L., Lauenborg J., Mathiesen E.R., Clausen T.D. Gestational diabetes mellitus and long-term consequences for mother and offspring: a view from Denmark. Diabetologia. 2016;59(7):1396–1399. - PubMed
-
- Metzger B.E., Lowe L.P., Dyer A.R., Trimble E.R., Chaovarindr U., Coustan D.R. Hyperglycemia and adverse pregnancy outcomes. New England Journal of Medicine. 2008;358(19):1991–2002. - PubMed
-
- Bellamy L., Casas J.P., Hingorani A.D., Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373(9677):1773–1779. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

