Direct effects of transcranial electric stimulation on brain circuits in rats and humans
- PMID: 29396478
- PMCID: PMC5797140
- DOI: 10.1038/s41467-018-02928-3
Direct effects of transcranial electric stimulation on brain circuits in rats and humans
Abstract
Transcranial electric stimulation is a non-invasive tool that can influence brain activity; however, the parameters necessary to affect local circuits in vivo remain to be explored. Here, we report that in rodents and human cadaver brains, ~75% of scalp-applied currents are attenuated by soft tissue and skull. Using intracellular and extracellular recordings in rats, we find that at least 1 mV/mm voltage gradient is necessary to affect neuronal spiking and subthreshold currents. We designed an 'intersectional short pulse' stimulation method to inject sufficiently high current intensities into the brain, while keeping the charge density and sensation on the scalp surface relatively low. We verify the regional specificity of this novel method in rodents; in humans, we demonstrate how it affects the amplitude of simultaneously recorded EEG alpha waves. Our combined results establish that neuronal circuits are instantaneously affected by intensity currents that are higher than those used in conventional protocols.
Conflict of interest statement
A.B. is the founder and owner of Amplipex and Evobrain LLCs, which manufacture biosignal amplifiers and stimulator devices. A patent application about the ISP method has been filed by A.B. and G.B. The remaining authors declare no competing financial interests.
Figures

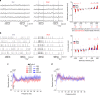

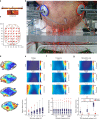

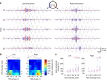


Comment in
-
Cross-Species Neuromodulation from High-Intensity Transcranial Electrical Stimulation.Trends Cogn Sci. 2018 May;22(5):372-374. doi: 10.1016/j.tics.2018.03.006. Epub 2018 Mar 27. Trends Cogn Sci. 2018. PMID: 29602532
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

