A multidrug ABC transporter with a taste for GTP
- PMID: 29396536
- PMCID: PMC5797166
- DOI: 10.1038/s41598-018-20558-z
A multidrug ABC transporter with a taste for GTP
Abstract
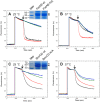
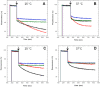
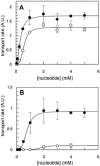
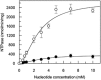
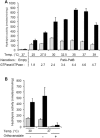
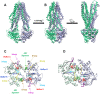
During the evolution of cellular bioenergetics, many protein families have been fashioned to match the availability and replenishment in energy supply. Molecular motors and primary transporters essentially need ATP to function while proteins involved in cell signaling or translation consume GTP. ATP-Binding Cassette (ABC) transporters are one of the largest families of membrane proteins gathering several medically relevant members that are typically powered by ATP hydrolysis. Here, a Streptococcus pneumoniae ABC transporter responsible for fluoroquinolones resistance in clinical settings, PatA/PatB, is shown to challenge this concept. It clearly favors GTP as the energy supply to expel drugs. This preference is correlated to its ability to hydrolyze GTP more efficiently than ATP, as found with PatA/PatB reconstituted in proteoliposomes or nanodiscs. Importantly, the ATP and GTP concentrations are similar in S. pneumoniae supporting the physiological relevance of GTP as the energy source of this bacterial transporter.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

