The RD-Connect Registry & Biobank Finder: a tool for sharing aggregated data and metadata among rare disease researchers
- PMID: 29396563
- PMCID: PMC5945774
- DOI: 10.1038/s41431-017-0085-z
The RD-Connect Registry & Biobank Finder: a tool for sharing aggregated data and metadata among rare disease researchers
Abstract
In rare disease (RD) research, there is a huge need to systematically collect biomaterials, phenotypic, and genomic data in a standardized way and to make them findable, accessible, interoperable and reusable (FAIR). RD-Connect is a 6 years global infrastructure project initiated in November 2012 that links genomic data with patient registries, biobanks, and clinical bioinformatics tools to create a central research resource for RDs. Here, we present RD-Connect Registry & Biobank Finder, a tool that helps RD researchers to find RD biobanks and registries and provide information on the availability and accessibility of content in each database. The finder concentrates information that is currently sparse on different repositories (inventories, websites, scientific journals, technical reports, etc.), including aggregated data and metadata from participating databases. Aggregated data provided by the finder, if appropriately checked, can be used by researchers who are trying to estimate the prevalence of a RD, to organize a clinical trial on a RD, or to estimate the volume of patients seen by different clinical centers. The finder is also a portal to other RD-Connect tools, providing a link to the RD-Connect Sample Catalogue, a large inventory of RD biological samples available in participating biobanks for RD research. There are several kinds of users and potential uses for the RD-Connect Registry & Biobank Finder, including researchers collaborating with academia and the industry, dealing with the questions of basic, translational, and/or clinical research. As of November 2017, the finder is populated with aggregated data for 222 registries and 21 biobanks.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

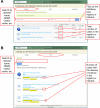

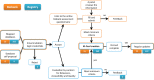
References
-
- Council of the European Union. Council Recommendation of 8 June 2009 on an action in the field of rare diseases (2009/c 151/02); Official Journal of the European Union 3/7/2009:C 151/7-10. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:C:2009:151:0007.... Accessed 20 Nov 2017.
-
- U.S. Government. 107th Congress Public Law 280. Rare Diseases Act of 2002. https://www.gpo.gov/fdsys/pkg/PLAW-107publ280/html/PLAW-107publ280.htm. Accessed 20 Nov 2017.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

