Effectiveness and safety of anticoagulants for the treatment of venous thromboembolism in patients with cancer
- PMID: 29396864
- PMCID: PMC5947542
- DOI: 10.1002/ajh.25059
Effectiveness and safety of anticoagulants for the treatment of venous thromboembolism in patients with cancer
Abstract
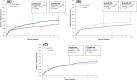
Anticoagulation is used to treat venous thromboembolism (VTE) in cancer patients, but may be associated with an increased risk of bleeding. VTE recurrence and major bleeding were assessed in cancer patients treated for VTE with the most currently prescribed anticoagulants in clinical practice. Newly diagnosed cancer patients (first VTE 1/1/2013-05/31/2015) who initiated rivaroxaban, low-molecular-weight heparin (LMWH), or warfarin were identified from Humana claims data and observed until end of eligibility or end of data availability. VTE recurrence was a hospitalization with a primary diagnosis of VTE ≥7 days after first VTE. Major bleeding events on treatment were identified using validated criteria. Cohorts were compared using Kaplan-Meier rates at 6 and 12 months and Cox proportional hazards models. Cohorts were adjusted for their differences at baseline. A total of 2428 patients (rivaroxaban: 707; LMWH: 660; warfarin: 1061) met inclusion criteria. Patient characteristics were well balanced after weighting. There was a trend for lower VTE recurrence rates in rivaroxaban users compared to LMWH users at 6 months (13.2% vs. 17.1%; P = .060) and significantly lower at 12 months (16.5% vs. 22.2%; P = .030) [HR: 0.72, 95% CI: (0.52-0.95); P = .024]. VTE recurrence rates were also lower for rivaroxaban than warfarin users at 6 months (13.2% vs. 17.5%; P = .014) and 12 months (15.7% vs. 19.9%; P = .017) [HR: 0.74, 95% CI: (0.56-0.96); P = .028]. Major bleeding rates were similar across cohorts. This real-world analysis suggests cancer patients with VTE treated with rivaroxaban had significantly lower risk of recurrent VTE and similar risk of bleeding compared to those treated with LMWH or warfarin.
© 2018 The Authors American Journal of Hematology Published by Wiley Periodicals, Inc.
Figures
Comment in
-
An answer to "anticoagulant treatment of cancer-associated venous thromboembolism: Interpreting real-world data with caution".Am J Hematol. 2018 Sep;93(9):E225-E227. doi: 10.1002/ajh.25165. Epub 2018 Aug 7. Am J Hematol. 2018. PMID: 29905381 No abstract available.
-
Anticoagulant treatment of cancer-associated venous thromboembolism: Interpreting real-world data with caution.Am J Hematol. 2018 Sep;93(9):E224-E225. doi: 10.1002/ajh.25164. Epub 2018 Aug 7. Am J Hematol. 2018. PMID: 30033546 No abstract available.
References
-
- Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5(3):632–634. - PubMed
-
- Lee AY, Levine MN. Venous thromboembolism and cancer: risks and outcomes. Circulation. 2003;107(23 Suppl 1):I17–I21. - PubMed
-
- Heit JA, Silverstein MD, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ. 3rd,. Risk factors for deep vein thrombosis and pulmonary embolism: a population‐based case‐control study. Arch Intern Med. 2000;160(6):809–815. - PubMed
-
- Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100(10):3484–3488. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous