Extended-release injectable naltrexone for opioid use disorder: a systematic review
- PMID: 29396985
- PMCID: PMC5993595
- DOI: 10.1111/add.14180
Extended-release injectable naltrexone for opioid use disorder: a systematic review
Abstract
Aims: To review systematically the published literature on extended-release naltrexone (XR-NTX, Vivitrol® ), marketed as a once-per-month injection product to treat opioid use disorder. We addressed the following questions: (1) how successful is induction on XR-NTX; (2) what are adherence rates to XR-NTX; and (3) does XR-NTX decrease opioid use? Factors associated with these outcomes as well as overdose rates were examined.
Methods: We searched PubMed and used Google Scholar for forward citation searches of peer-reviewed papers from January 2006 to June 2017. Studies that included individuals seeking treatment for opioid use disorder who were offered XR-NTX were included.
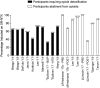
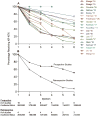
Results: We identified and included 34 studies. Pooled estimates showed that XR-NTX induction success was lower in studies that included individuals that required opioid detoxification [62.6%, 95% confidence interval (CI) = 54.5-70.0%] compared with studies that included individuals already detoxified from opioids (85.0%, 95% CI = 78.0-90.1%); 44.2% (95% CI = 33.1-55.9%) of individuals took all scheduled injections of XR-NTX, which were usually six or fewer. Adherence was higher in prospective investigational studies (i.e. studies conducted in a research context according to a study protocol) compared to retrospective studies of medical records taken from routine care (6-month rates: 46.7%, 95% CI = 34.5-59.2% versus 10.5%, 95% CI = 4.6-22.4%, respectively). Compared with referral to treatment, XR-NTX reduced opioid use in adults under criminal justice supervision and when administered to inmates before release. XR-NTX reduced opioid use compared with placebo in Russian adults, but this effect was confounded by differential retention between study groups. XR-NTX showed similar efficacy to buprenorphine when randomization occurred after detoxification, but was inferior to buprenorphine when randomization occurred prior to detoxification.
Conclusions: Many individuals intending to start extended-release naltrexone (XR-NTX) do not and most who do start XR-NTX discontinue treatment prematurely, two factors that limit its clinical utility significantly. XR-NTX appears to decrease opioid use but there are few experimental demonstrations of this effect.
Keywords: Extended-release; heroin; injectable; medication-assisted treatment; naltrexone; opioid use disorder; prescription opioids.
© 2018 Society for the Study of Addiction.
Conflict of interest statement
Figures

Comment in
-
Long-acting naltrexone has long-acting benefits and 100% induction rates are not difficult to achieve.Addiction. 2019 Jan;114(1):188-189. doi: 10.1111/add.14448. Epub 2018 Oct 30. Addiction. 2019. PMID: 30345573 No abstract available.
-
Extended-release injectable naltrexone (XR-NTX): a response to clinical issues raised by Brewer & Streel.Addiction. 2019 Jan;114(1):189-190. doi: 10.1111/add.14462. Epub 2018 Oct 30. Addiction. 2019. PMID: 30345640 No abstract available.
References
-
- Degenhardt L, Charlson F, Mathers B, Hall WD, Flaxman AD, Johns N, et al. The global epidemiology and burden of opioid dependence: Results from the global burden of disease 2010 study. Addiction. 2014;109:1320–1333. - PubMed
-
- Han B, Compton WM, Jones CM, Cai R. Nonmedical prescription opioid use and use disorders among adults aged 18 through 64 years in the United States, 2003–2013. JAMA. 2015;314:1468–1478. - PubMed
-
- Frank RG, Pollack HA. Addressing the fentanyl threat to public health. N Engl J Med. 2017;376:605–607. - PubMed
-
- Murthy VH. Ending the opioid epidemic—A call to action. N Engl J Med. 2016;375:2413–2415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

