The dichotomous role of H2S in cancer cell biology? Déjà vu all over again
- PMID: 29397935
- PMCID: PMC5866221
- DOI: 10.1016/j.bcp.2018.01.042
The dichotomous role of H2S in cancer cell biology? Déjà vu all over again
Abstract
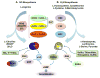
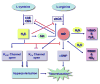
Nitric oxide (NO) a gaseous free radical is one of the ten smallest molecules found in nature, while hydrogen sulfide (H2S) is a gas that bears the pungent smell of rotten eggs. Both are toxic yet they are gasotransmitters of physiological relevance. There appears to be an uncanny resemblance between the general actions of these two gasotransmitters in health and disease. The role of NO and H2S in cancer has been quite perplexing, as both tumor promotion and inflammatory activities as well as anti-tumor and antiinflammatory properties have been described. These paradoxes have been explained for both gasotransmitters in terms of each having a dual or biphasic effect that is dependent on the local flux of each gas. In this review/commentary, I have discussed the major roles of NO and H2S in carcinogenesis, evaluating their dual nature, focusing on the enzymes that contribute to this paradox and evaluate the pros and cons of inhibiting or inducing each of these enzymes.
Keywords: CBS; CSE; Carcinogenesis; Hydrogen sulfide; Nitric oxide; iNOS.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
KK holds an equity position in Avicenna Pharmaceuticals, Inc. to which NOSH-NSAIDs briefly described in this review have been licensed.
Figures
References
-
- Hattori Y, Kasai K, Gross SS. NO suppresses while peroxynitrite sustains NF-kappaB: a paradigm to rationalize cytoprotective and cytotoxic actions attributed to NO. Cardiovasc Res. 2004;63:31–40. - PubMed
-
- Katsuyama K, Shichiri M, Marumo F, Hirata Y. NO inhibits cytokine-induced iNOS expression and NF-kappaB activation by interfering with phosphorylation and degradation of IkappaB-alpha. Arterioscler Thromb Vasc Biol. 1998;18:1796–802. - PubMed
-
- Hogaboam CM, Befus AD, Wallace JL. Modulation of rat mast cell reactivity by IL-1 beta. Divergent effects on nitric oxide and platelet-activating factor release. J Immunol. 1993;151:3767–74. - PubMed
-
- Huang JQ, Sridhar S, Chen Y, Hunt RH. Meta-analysis of the relationship between helicobacter pylori seropositivity and gastric cancer. Gastroenterology. 1998;114:1169–79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

