Dopamine Secretion Is Mediated by Sparse Active Zone-like Release Sites
- PMID: 29398114
- PMCID: PMC5807134
- DOI: 10.1016/j.cell.2018.01.008
Dopamine Secretion Is Mediated by Sparse Active Zone-like Release Sites
Abstract
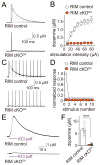
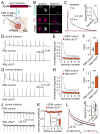
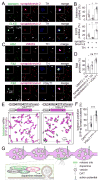
Dopamine controls essential brain functions through volume transmission. Different from fast synaptic transmission, where neurotransmitter release and receptor activation are tightly coupled by an active zone, dopamine transmission is widespread and may not necessitate these organized release sites. Here, we determine whether striatal dopamine secretion employs specialized machinery for release. Using super resolution microscopy, we identified co-clustering of the active zone scaffolding proteins bassoon, RIM and ELKS in ∼30% of dopamine varicosities. Conditional RIM knockout disrupted this scaffold and, unexpectedly, abolished dopamine release, while ELKS knockout had no effect. Optogenetic experiments revealed that dopamine release was fast and had a high release probability, indicating the presence of protein scaffolds for coupling Ca2+ influx to vesicle fusion. Hence, dopamine secretion is mediated by sparse, mechanistically specialized active zone-like release sites. This architecture supports spatially and temporally precise coding for dopamine and provides molecular machinery for regulation.
Keywords: ELKS; RIM; active zone; bassoon; dopamine; exocytosis; striatum; superresolution; varicosity; volume transmission.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures




Comment in
-
To Bind or Not to Bind: Unravelling GPCR Polypharmacology.Cell. 2018 Feb 8;172(4):636-638. doi: 10.1016/j.cell.2018.01.018. Cell. 2018. PMID: 29425482
-
Synaptic transmission: A hare as well as a tortoise.Nat Rev Neurosci. 2018 Apr;19(4):183. doi: 10.1038/nrn.2018.17. Epub 2018 Feb 15. Nat Rev Neurosci. 2018. PMID: 29467467 No abstract available.
Similar articles
-
The active zone protein family ELKS supports Ca2+ influx at nerve terminals of inhibitory hippocampal neurons.J Neurosci. 2014 Sep 10;34(37):12289-303. doi: 10.1523/JNEUROSCI.0999-14.2014. J Neurosci. 2014. PMID: 25209271 Free PMC article.
-
Molecular and functional architecture of striatal dopamine release sites.Neuron. 2022 Jan 19;110(2):248-265.e9. doi: 10.1016/j.neuron.2021.10.028. Epub 2021 Nov 11. Neuron. 2022. PMID: 34767769 Free PMC article.
-
Fusion Competent Synaptic Vesicles Persist upon Active Zone Disruption and Loss of Vesicle Docking.Neuron. 2016 Aug 17;91(4):777-791. doi: 10.1016/j.neuron.2016.07.005. Neuron. 2016. PMID: 27537483 Free PMC article.
-
Role of the active zone protein, ELKS, in insulin secretion from pancreatic β-cells.Mol Metab. 2019 Sep;27S(Suppl):S81-S91. doi: 10.1016/j.molmet.2019.06.017. Mol Metab. 2019. PMID: 31500835 Free PMC article. Review.
-
Molecular organization of the presynaptic active zone.Cell Tissue Res. 2006 Nov;326(2):379-91. doi: 10.1007/s00441-006-0244-y. Epub 2006 Jul 25. Cell Tissue Res. 2006. PMID: 16865347 Review.
Cited by
-
Designing a norepinephrine optical tracer for imaging individual noradrenergic synapses and their activity in vivo.Nat Commun. 2018 Jul 19;9(1):2838. doi: 10.1038/s41467-018-05075-x. Nat Commun. 2018. PMID: 30026491 Free PMC article.
-
Unraveling the dynamics of dopamine release and its actions on target cells.Trends Neurosci. 2023 Mar;46(3):228-239. doi: 10.1016/j.tins.2022.12.005. Epub 2023 Jan 10. Trends Neurosci. 2023. PMID: 36635111 Free PMC article. Review.
-
Responses and functions of dopamine in nucleus accumbens core during social behaviors.Cell Rep. 2022 Aug 23;40(8):111246. doi: 10.1016/j.celrep.2022.111246. Cell Rep. 2022. PMID: 36001967 Free PMC article.
-
Prenatal hypoxia alters the early ontogeny of dopamine neurons.Transl Psychiatry. 2022 Jun 7;12(1):238. doi: 10.1038/s41398-022-02005-w. Transl Psychiatry. 2022. PMID: 35672280 Free PMC article.
-
Carbon Monoxide, a Retrograde Messenger Generated in Postsynaptic Mushroom Body Neurons, Evokes Noncanonical Dopamine Release.J Neurosci. 2020 Apr 29;40(18):3533-3548. doi: 10.1523/JNEUROSCI.2378-19.2020. Epub 2020 Apr 6. J Neurosci. 2020. PMID: 32253360 Free PMC article.
References
-
- Acuna C, Liu X, Gonzalez A, Südhof TC. RIM-BPs Mediate Tight Coupling of Action Potentials to Ca2+-Triggered Neurotransmitter Release. Neuron. 2015;87:1234–1247. - PubMed
-
- Acuna C, Liu X, Südhof TC. How to Make an Active Zone: Unexpected Universal Functional Redundancy between RIMs and RIM-BPs. Neuron. 2016;91:792–807. - PubMed
-
- Agnati LF, Zoli M, Strömberg I, Fuxe K. Intercellular communication in the brain: Wiring versus volume transmission. Neuroscience. 1995;69:711–726. - PubMed
-
- Altrock WD, tom Dieck S, Sokolov M, Meyer AC, Sigler A, Brakebusch C, Fässler R, Richter K, Boeckers TM, Potschka H, et al. Functional inactivation of a fraction of excitatory synapses in mice deficient for the active zone protein bassoon. Neuron. 2003;37:787–800. - PubMed
-
- Augustin I, Rosenmund C, Sudhof TC, Brose N. Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature. 1999;400:457–461. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

