Self-Organized Attractor Dynamics in the Developing Head Direction Circuit
- PMID: 29398220
- PMCID: PMC5835142
- DOI: 10.1016/j.cub.2018.01.010
Self-Organized Attractor Dynamics in the Developing Head Direction Circuit
Abstract
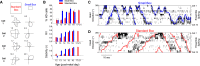
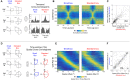
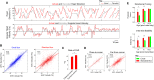
Head direction (HD) cells are neurons found in an extended cortical and subcortical network that signal the orientation of an animal's head relative to its environment [1-3]. They are a fundamental component of the wider circuit of spatially responsive hippocampal formation neurons that make up the neural cognitive map of space [4]. During post-natal development, HD cells are the first among spatially modulated neurons in the hippocampal circuit to exhibit mature firing properties [5, 6], but before eye opening, HD cell responses in rat pups have low directional information and are directionally unstable [7, 8]. Using Bayesian decoding of HD cell ensemble activity recorded in the anterodorsal thalamic nucleus (ADN), we characterize this instability and identify its source: under-signaling of angular head velocity, which incompletely shifts the directional signal in proportion to head turns. We find evidence that geometric cues (the corners of a square environment) can be used to mitigate this under-signaling and, thereby, stabilize the directional signal even before eye opening. Crucially, even when directional firing cannot be stabilized, ensembles of unstable HD cells show short-timescale (1-10 s) temporal and spatial couplings consistent with an adult-like HD network. The HD network is widely modeled as a continuous attractor whose output is one coherent activity peak, updated during movement by angular head velocity signals and anchored by landmark cues [9-11]. Our findings present strong evidence for this model, and they demonstrate that the required network circuitry is in place and functional early during development, independent of reference to landmark information.
Keywords: anterodorsal thalamic nucleus; attractor network; development; head direction cells.
Copyright © 2018 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures

References
-
- Taube J.S. The head direction signal: origins and sensory-motor integration. Annu. Rev. Neurosci. 2007;30:181–207. - PubMed
-
- Finkelstein A., Derdikman D., Rubin A., Foerster J.N., Las L., Ulanovsky N. Three-dimensional head-direction coding in the bat brain. Nature. 2015;517:159–164. - PubMed
-
- O’Keefe J., Nadel L. Oxford University Press; 1978. The Hippocampus as a Cognitive Map.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

