Graded Arrays of Spinal and Supraspinal V2a Interneuron Subtypes Underlie Forelimb and Hindlimb Motor Control
- PMID: 29398364
- PMCID: PMC8601153
- DOI: 10.1016/j.neuron.2018.01.023
Graded Arrays of Spinal and Supraspinal V2a Interneuron Subtypes Underlie Forelimb and Hindlimb Motor Control
Abstract
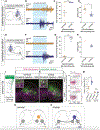
The spinal cord contains neural networks that enable regionally distinct motor outputs along the body axis. Nevertheless, it remains unclear how segment-specific motor computations are processed because the cardinal interneuron classes that control motor neurons appear uniform at each level of the spinal cord. V2a interneurons are essential to both forelimb and hindlimb movements, and here we identify two major types that emerge during development: type I neurons marked by high Chx10 form recurrent networks with neighboring spinal neurons and type II neurons that downregulate Chx10 and project to supraspinal structures. Types I and II V2a interneurons are arrayed in counter-gradients, and this network activates different patterns of motor output at cervical and lumbar levels. Single-cell RNA sequencing (RNA-seq) revealed type I and II V2a neurons are each comprised of multiple subtypes. Our findings uncover a molecular and anatomical organization of V2a interneurons reminiscent of the orderly way motor neurons are divided into columns and pools.
Keywords: V2a interneuron; excitatory neuron; forelimb; hindlimb; locomotion; motor control; neuronal diversity; optogenetics; single-cell RNA-seq; spinal cord.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures

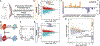




References
-
- Al-Mosawie A, Wilson JM, and Brownstone RM (2007). Heterogeneity of V2-derived interneurons in the adult mouse spinal cord. Eur. J. Neurosci. 26, 3003–3015. - PubMed
-
- Alstermark B, Isa T, Pettersson LG, and Sasaki S (2007). The C3-C4 propriospinal system in the cat and monkey: a spinal pre-motoneuronal centre for voluntary motor control. Acta Physiol. (Oxf.) 189, 123–140. - PubMed
-
- Arber S (2012). Motor circuits in action: specification, connectivity, and function. Neuron 74, 975–989. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

