RHOA G17V Induces T Follicular Helper Cell Specification and Promotes Lymphomagenesis
- PMID: 29398449
- PMCID: PMC5811310
- DOI: 10.1016/j.ccell.2018.01.001
RHOA G17V Induces T Follicular Helper Cell Specification and Promotes Lymphomagenesis
Abstract
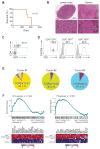
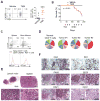
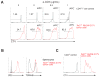
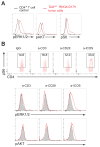
Angioimmunoblastic T cell lymphoma (AITL) is an aggressive tumor derived from malignant transformation of T follicular helper (Tfh) cells. AITL is characterized by loss-of-function mutations in Ten-Eleven Translocation 2 (TET2) epigenetic tumor suppressor and a highly recurrent mutation (p.Gly17Val) in the RHOA small GTPase. Yet, the specific role of RHOA G17V in AITL remains unknown. Expression of Rhoa G17V in CD4+ T cells induces Tfh cell specification; increased proliferation associated with inducible co-stimulator (ICOS) upregulation and increased phosphoinositide 3-kinase (PI3K) and mitogen-activated protein kinase signaling. Moreover, RHOA G17V expression together with Tet2 loss resulted in development of AITL in mice. Importantly, Tet2-/-RHOA G17V tumor proliferation in vivo can be inhibited by ICOS/PI3K-specific blockade, supporting a driving role for ICOS signaling in Tfh cell transformation.
Keywords: ICOS; RHOA G17V; T follicular helper cells; TET2; angioimmunoblastic T cell lymphoma.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Aicher A, Hayden-Ledbetter M, Brady WA, Pezzutto A, Richter G, Magaletti D, Buckwalter S, Ledbetter JA, Clark EA. Characterization of human inducible costimulator ligand expression and function. J Immunol. 2000;164:4689–4696. - PubMed
-
- Borroto A, Gil D, Delgado P, Vicente-Manzanares M, Alcover A, Sanchez-Madrid F, Alarcon B. Rho regulates T cell receptor ITAM-induced lymphocyte spreading in an integrin-independent manner. Eur J Immunol. 2000;30:3403–3410. - PubMed
-
- Boulter E, Estrach S, Garcia-Mata R, Feral CC. Off the beaten paths: alternative and crosstalk regulation of Rho GTPases. FASEB J. 2012;26:469–479. - PubMed
-
- Burmeister Y, Lischke T, Dahler AC, Mages HW, Lam KP, Coyle AJ, Kroczek RA, Hutloff A. ICOS controls the pool size of effector-memory and regulatory T cells. J Immunol. 2008;180:774–782. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

