Clinical pharmacology of intravitreal anti-VEGF drugs
- PMID: 29398697
- PMCID: PMC5997665
- DOI: 10.1038/s41433-018-0021-7
Clinical pharmacology of intravitreal anti-VEGF drugs
Abstract
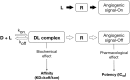
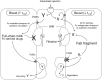
Clinical efficacy of intravitreal anti-VEGF drugs has been widely demonstrated in several angiogenesis-driven eye diseases including diabetic macular edema and the neovascular form of age-related macular degeneration. Pegaptanib, ranibizumab, and aflibercept have been approved for use in the eye, whereas bevacizumab is widely used by ophthalmologists to treat patients "off-label". These drugs are active in the nanomolar to picomolar range; however, caution is required when establishing the rank order of affinity and potency due to in vitro inter-experimental variation. Despite the small doses used for eye diseases and the intravitreal route of administration may limit systemic side effects, these drugs can penetrate into blood circulation and alter systemic VEGF with unknown clinical consequences, particularly in vulnerable groups of patients. Clinical pharmacokinetics of ocular anti-VEGF agents should therefore be taken into account when choosing the right drug for the individual patient. The gaps in current understanding that leave open important questions are as follows: (i) uncertainty about which drug should be given first, (ii) how long these drugs can be used safely, and (iii) the choice of the best pharmacological strategy after first-line treatment failure. The current review article, based on the information published in peer-reviewed published papers relevant to anti-VEGF treatments and available on the PubMed database, describes in detail the clinical pharmacology of this class of drugs to provide a sound pharmacological basis for their proper use in ophthalmology clinical practice.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

