Effects of ginkgol C17:1 on cisplatin-induced autophagy and apoptosis in HepG2 cells
- PMID: 29399162
- PMCID: PMC5772831
- DOI: 10.3892/ol.2017.7398
Effects of ginkgol C17:1 on cisplatin-induced autophagy and apoptosis in HepG2 cells
Abstract
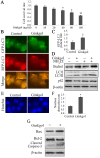
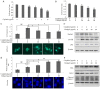
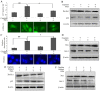
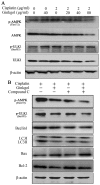
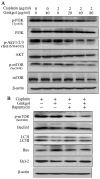
Previous studies have demonstrated that ginkgol C17:1 significantly inhibits human liver cancer cells and enhances the anticancer activity of cisplatin in vivo and in vitro. However, the mechanism and biological function of ginkgol C17:1 on cells undergoing chemotherapy remain unclear. The aim of the present study was to determine the antitumor activity and mechanism of ginkgol C17:1 in combination with cisplatin in human hepatoblastoma HepG2 cells. The green fluorescent protein (GFP)-light chain 3 (LC3) adenovirus was transfected into HepG2 cells and autophagic flux was determined using fluorescence microscopy. Western blot analysis was also conducted to measure the expression of proteins associated with apoptosis, autophagy and their associated signaling pathways. Compared with the control group, autophagic flux and nucleus aberration rates were significantly increased (P<0.05), and the expression of proteins associated with autophagy and apoptosis were increased in the groups treated with cisplatin or ginkgol C17:1, respectively. However, following co-treatment with ginkgol C17:1 and cisplatin, the autophagic flux and the expression of autophagy proteins decreased; however, the nucleus aberration rate and apoptosis protein expression significantly increased (P<0.05) compared with the group treated with cisplatin alone. Additionally, the signaling pathways of autophagy and apoptosis were also activated following treatment with cisplatin, alone and in combination with ginkgol C17:1. Taken together, these results indicate that ginkgol C17:1 inhibits cisplatin-induced autophagy via AMP-activated protein kinase/ULK1signaling and increases cisplatin-induced apoptosis in HepG2 cells via the phosphoinositide 3-kinase/Akt/mechanistic target of rapamycin pathway.
Keywords: HepG2 cells; apoptosis; autophagy; cisplatin; ginkgol C17:1.
Figures
Similar articles
-
Differential effects of ginkgol C17:1 on cisplatin-induced cytotoxicity: Protecting human normal L02 hepatocytes versus sensitizing human hepatoma HepG2 cells.Oncol Lett. 2019 Mar;17(3):3181-3190. doi: 10.3892/ol.2019.9974. Epub 2019 Jan 25. Oncol Lett. 2019. PMID: 30867748 Free PMC article.
-
Ginkgol C17:1 inhibits tumor growth by blunting the EGF- PI3K/Akt signaling pathway.J Biomed Res. 2017 Jan 19;31(3):232-239. doi: 10.7555/JBR.31.20160039. J Biomed Res. 2017. PMID: 28808215 Free PMC article.
-
Inhibitory effect of Ginkgol C17:1 on the biological behavior of tumor cells.Oncol Lett. 2017 Mar;13(3):1873-1879. doi: 10.3892/ol.2017.5664. Epub 2017 Feb 1. Oncol Lett. 2017. PMID: 28454337 Free PMC article.
-
Extraction, Purification, and Elucidation of Six Ginkgol Homologs from Ginkgo biloba Sarcotesta and Evaluation of Their Anticancer Activities.Molecules. 2022 Nov 11;27(22):7777. doi: 10.3390/molecules27227777. Molecules. 2022. PMID: 36431878 Free PMC article.
-
Diosmetin inhibits cell proliferation and induces apoptosis by regulating autophagy via the mammalian target of rapamycin pathway in hepatocellular carcinoma HepG2 cells.Oncol Lett. 2016 Dec;12(6):4385-4392. doi: 10.3892/ol.2016.5301. Epub 2016 Oct 19. Oncol Lett. 2016. PMID: 28101201 Free PMC article.
Cited by
-
Advances in the conventional clinical treatment for hepatoblastoma and therapeutic innovation.World J Pediatr Surg. 2021 Jun 8;4(3):e000220. doi: 10.1136/wjps-2020-000220. eCollection 2021. World J Pediatr Surg. 2021. PMID: 36474976 Free PMC article. Review.
-
Differential effects of ginkgol C17:1 on cisplatin-induced cytotoxicity: Protecting human normal L02 hepatocytes versus sensitizing human hepatoma HepG2 cells.Oncol Lett. 2019 Mar;17(3):3181-3190. doi: 10.3892/ol.2019.9974. Epub 2019 Jan 25. Oncol Lett. 2019. PMID: 30867748 Free PMC article.
-
SUMOylation regulates the aggressiveness of breast cancer-associated fibroblasts.Cell Oncol (Dordr). 2025 Apr;48(2):437-453. doi: 10.1007/s13402-024-01005-w. Epub 2024 Oct 21. Cell Oncol (Dordr). 2025. PMID: 39432155 Free PMC article.
-
Plant-derived natural products and combination therapy in liver cancer.Front Oncol. 2023 Feb 14;13:1116532. doi: 10.3389/fonc.2023.1116532. eCollection 2023. Front Oncol. 2023. PMID: 36865794 Free PMC article. Review.
-
Inhibiting SUMO1-mediated SUMOylation induces autophagy-mediated cancer cell death and reduces tumour cell invasion via RAC1.J Cell Sci. 2019 Oct 22;132(20):jcs234120. doi: 10.1242/jcs.234120. J Cell Sci. 2019. PMID: 31578236 Free PMC article.
References
-
- Pang RTK, Poon TCW, Wong N, Lai PSB, Wong NLY, Chan CML, Yu JWS, Chan ATC, Sung JJY. Comparison of protein expression patterns between hepatocellular carcinoma cell lines and a hepatoblastoma cell line. Clin Proteomics. 2004;1:313–331. doi: 10.1385/CP:1:3-4:313. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
