A safe and highly efficient tumor-targeted type I interferon immunotherapy depends on the tumor microenvironment
- PMID: 29399401
- PMCID: PMC5790344
- DOI: 10.1080/2162402X.2017.1398876
A safe and highly efficient tumor-targeted type I interferon immunotherapy depends on the tumor microenvironment
Abstract
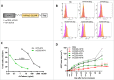
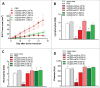
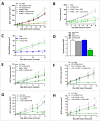
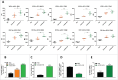
Despite approval for the treatment of various malignancies, clinical application of cytokines such as type I interferon (IFN) is severely impeded by their systemic toxicity. AcTakines (Activity-on-Target cytokines) are optimized immunocytokines that, when injected in mice, only reveal their activity upon cell-specific impact. We here show that type I IFN-derived AcTaferon targeted to the tumor displays strong antitumor activity without any associated toxicity, in contrast with wild type IFN. Treatment with CD20-targeted AcTaferon of CD20+ lymphoma tumors or melanoma tumors engineered to be CD20+, drastically reduced tumor growth. This antitumor effect was completely lost in IFNAR- or Batf3-deficient mice, and depended on IFN signaling in conventional dendritic cells. Also the presence of, but not the IFN signaling in, CD8+ T lymphocytes was critical for proficient antitumor effects. When combined with immunogenic chemotherapy, low-dose TNF, or immune checkpoint blockade strategies such as anti-PDL1, anti-CTLA4 or anti-LAG3, complete tumor regressions and subsequent immunity (memory) were observed, still without any concomitant morbidity, again in sharp contrast with wild type IFN. Interestingly, the combination therapy of tumor-targeted AcTaferon with checkpoint inhibiting antibodies indicated its ability to convert nonresponding tumors into responders. Collectively, our findings demonstrate that AcTaferon targeted to tumor-specific surface markers may provide a safe and generic addition to cancer (immuno)therapies.
Keywords: AcTaferon; Immunotherapy; checkpoint inhibitors; dendritic cells; engineered immunocytokine; targeting; toxicity; tumor microenvironment; type I interferon.
Figures


Similar articles
-
Delivering Type I Interferon to Dendritic Cells Empowers Tumor Eradication and Immune Combination Treatments.Cancer Res. 2018 Jan 15;78(2):463-474. doi: 10.1158/0008-5472.CAN-17-1980. Epub 2017 Nov 29. Cancer Res. 2018. PMID: 29187401
-
Engineering Newcastle Disease Virus as an Oncolytic Vector for Intratumoral Delivery of Immune Checkpoint Inhibitors and Immunocytokines.J Virol. 2020 Jan 17;94(3):e01677-19. doi: 10.1128/JVI.01677-19. Print 2020 Jan 17. J Virol. 2020. PMID: 31694938 Free PMC article.
-
Co-administration of RANKL and CTLA4 Antibodies Enhances Lymphocyte-Mediated Antitumor Immunity in Mice.Clin Cancer Res. 2017 Oct 1;23(19):5789-5801. doi: 10.1158/1078-0432.CCR-17-0606. Epub 2017 Jun 20. Clin Cancer Res. 2017. PMID: 28634284
-
CD8+ cytotoxic T lymphocytes in cancer immunotherapy: A review.J Cell Physiol. 2019 Jun;234(6):8509-8521. doi: 10.1002/jcp.27782. Epub 2018 Nov 22. J Cell Physiol. 2019. PMID: 30520029 Review.
-
The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma.Clin Ther. 2015 Apr 1;37(4):764-82. doi: 10.1016/j.clinthera.2015.02.018. Epub 2015 Mar 29. Clin Ther. 2015. PMID: 25823918 Free PMC article. Review.
Cited by
-
Direct and indirect anti-leukemic properties of activity-on-target interferons for the treatment of T-cell acute lymphoblastic leukemia.Haematologica. 2022 Jun 1;107(6):1448-1453. doi: 10.3324/haematol.2021.278913. Haematologica. 2022. PMID: 34647441 Free PMC article. No abstract available.
-
Chemotherapeutic and targeted drugs-induced immunogenic cell death in cancer models and antitumor therapy: An update review.Front Pharmacol. 2023 Apr 21;14:1152934. doi: 10.3389/fphar.2023.1152934. eCollection 2023. Front Pharmacol. 2023. PMID: 37153795 Free PMC article. Review.
-
Utilizing Immunocytokines for Cancer Therapy.Antibodies (Basel). 2021 Mar 9;10(1):10. doi: 10.3390/antib10010010. Antibodies (Basel). 2021. PMID: 33803078 Free PMC article. Review.
-
Safe eradication of large established tumors using neovasculature-targeted tumor necrosis factor-based therapies.EMBO Mol Med. 2020 Feb 7;12(2):e11223. doi: 10.15252/emmm.201911223. Epub 2020 Jan 8. EMBO Mol Med. 2020. PMID: 31912630 Free PMC article.
-
Development of T-cell engagers selective for cells co-expressing two antigens.MAbs. 2022 Jan-Dec;14(1):2115213. doi: 10.1080/19420862.2022.2115213. MAbs. 2022. PMID: 36206404 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
