Oncolytic viruses sensitize human tumor cells for NY-ESO-1 tumor antigen recognition by CD4+ effector T cells
- PMID: 29399408
- PMCID: PMC5790392
- DOI: 10.1080/2162402X.2017.1407897
Oncolytic viruses sensitize human tumor cells for NY-ESO-1 tumor antigen recognition by CD4+ effector T cells
Abstract
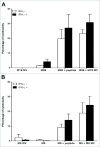
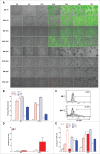
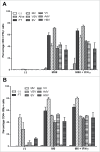
Oncolytic immunotherapy using oncolytic viruses (OV) has been shown to stimulate the antitumor immune response by inducing the release of tumor-associated antigens (TAA) and danger signals from the dying infected tumor cells. In this study, we sought to determine if the lysis of tumor cells induced by different OV: measles virus, vaccinia virus, vesicular stomatitis virus, herpes simplex type I virus, adenovirus or enterovirus, has consequences on the capacity of tumor cells to present TAA, such as NY-ESO-1. We show that the co-culture of NY-ESO-1neg/HLA-DP4pos melanoma cells with NY-ESO-1pos/HLA-DP4neg melanoma cells infected and killed by different OV induces an intercellular transfer of NY-ESO-1 that allows the recognition of NY-ESO-1neg/HLA-DP4pos tumor cells by an HLA-DP4/NY-ESO-1(157-170)-specific CD4+ cytotoxic T cell clone, NY67. We then confirmed this result in a second model with an HLA-DP4+ melanoma cell line that expresses a low amount of NY-ESO-1. Recognition of this cell line by the NY67 clone is largely increased in the presence of OV productive infection. Altogether, our results show for the first time another mechanism of stimulation of the anti-tumor immune response by OV, via the loading of tumor cells with TAA that sensitizes them for direct recognition by specific effector CD4+ T cells, supporting the use of OV for cancer immunotherapy.
Keywords: CD4+ T Lymphocytes; Melanoma; Oncolytic Viruses; Oncolytic immunotherapy; Tumor-Associated Antigens.
Figures





References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
