Anti-fouling strategies for central venous catheters
- PMID: 29399528
- PMCID: PMC5778515
- DOI: 10.21037/cdt.2017.09.18
Anti-fouling strategies for central venous catheters
Abstract
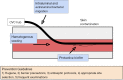
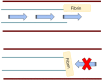
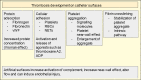
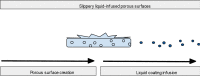
Central venous catheters (CVCs) are ubiquitous in the healthcare industry and carry two common complications, catheter related infections and occlusion, particularly by thrombus. Catheter-related bloodstream infections (CRBSI) are an important cause of nosocomial infections that increase patient morbidity, mortality, and hospital cost. Innovative design strategies for intravenous catheters can help reduce these preventable infections. Antimicrobial coatings can play a major role in preventing disease. These coatings can be divided into two major categories: drug eluting and non-drug eluting. Much of these catheter designs are targeted at preventing the formation of microbial biofilms that make treatment of CRBSI nearly impossible without removal of the intravenous device. Exciting developments in catheter impregnation with antibiotics as well as nanoscale surface design promise innovative changes in the way that physicians manage intravenous catheters. Occlusion of a catheter renders the catheter unusable and is often treated by tissue plasminogen activator administration or replacement of the line. Prevention of this complication requires a thorough understanding of the mechanisms of platelet aggregation, signaling and cross-linking. This article will look at the advances in biomaterial design specifically drug eluting, non-drug eluting, lubricious coatings and micropatterning as well as some of the characteristics of each as they relate to CVCs.
Keywords: Biofouling; catheters; central venous catheters (CVCs); infections; thrombosis.
Conflict of interest statement
Conflicts of Interest: The authors have no conflicts of interest to declare.
Figures

Similar articles
-
Antibiotic lock therapy for salvage of tunneled central venous catheters with catheter colonization and catheter-related bloodstream infection.Transpl Infect Dis. 2019 Feb;21(1):e13017. doi: 10.1111/tid.13017. Epub 2018 Nov 19. Transpl Infect Dis. 2019. PMID: 30369006
-
Five-Lumen Antibiotic-Impregnated Femoral Central Venous Catheters in Severely Burned Patients: An Investigation of Device Utility and Catheter-Related Bloodstream Infection Rates.J Burn Care Res. 2015 Jul-Aug;36(4):493-9. doi: 10.1097/BCR.0000000000000186. J Burn Care Res. 2015. PMID: 25407386
-
Surface-treated catheters--a review.Semin Dial. 2008 Nov-Dec;21(6):542-6. doi: 10.1111/j.1525-139X.2008.00499.x. Epub 2008 Sep 24. Semin Dial. 2008. PMID: 19000120 Review.
-
Administration of taurolidine-citrate lock solution for prevention of central venous catheter infection in adult neutropenic haematological patients: a randomised, double-blinded, placebo-controlled trial (TAURCAT).Trials. 2018 May 2;19(1):264. doi: 10.1186/s13063-018-2647-y. Trials. 2018. PMID: 29720244 Free PMC article.
-
Clinical review: new technologies for prevention of intravascular catheter-related infections.Crit Care. 2004 Jun;8(3):157-62. doi: 10.1186/cc2380. Epub 2003 Sep 29. Crit Care. 2004. PMID: 15153233 Free PMC article. Review.
Cited by
-
Prevention of uropathogenic E. coli biofilm formation by hydrophobic nanoparticle coatings on polymeric substrates.RSC Appl Interfaces. 2024 Mar 20;1(4):667-670. doi: 10.1039/d3lf00241a. eCollection 2024 Jul 9. RSC Appl Interfaces. 2024. PMID: 38988413 Free PMC article.
-
Minimising Blood Stream Infection: Developing New Materials for Intravascular Catheters.Medicines (Basel). 2020 Aug 26;7(9):49. doi: 10.3390/medicines7090049. Medicines (Basel). 2020. PMID: 32858838 Free PMC article. Review.
-
Modulating the foreign body response of implants for diabetes treatment.Adv Drug Deliv Rev. 2021 Jul;174:87-113. doi: 10.1016/j.addr.2021.01.011. Epub 2021 Jan 21. Adv Drug Deliv Rev. 2021. PMID: 33484736 Free PMC article. Review.
-
Inhibition of bacterial attachment and biofilm formation by a novel intravenous catheter material using an in vitro percutaneous catheter insertion model.Med Devices (Auckl). 2018 Dec 19;11:427-432. doi: 10.2147/MDER.S183409. eCollection 2018. Med Devices (Auckl). 2018. PMID: 30588133 Free PMC article.
-
Biogenic silver nanoparticles (AgNPs) from Tinosporacordifolia leaves: An effective antibiofilm agent against Staphylococcus aureus ATCC 23235.Front Chem. 2023 Mar 7;11:1118454. doi: 10.3389/fchem.2023.1118454. eCollection 2023. Front Chem. 2023. PMID: 36959877 Free PMC article.
References
-
- O'Grady NP, Alexander M, Dellinger EP, et al. Guidelines for the prevention of intravascular catheter-related infections. Centers for disease control and prevention. MMWR Recomm Rep 2002;51:1-29. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
