Calcium-dependent protein kinases are potential targets for Toxoplasma gondii vaccine
- PMID: 29399577
- PMCID: PMC5795042
- DOI: 10.7774/cevr.2018.7.1.24
Calcium-dependent protein kinases are potential targets for Toxoplasma gondii vaccine
Abstract
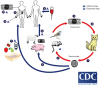
Toxoplasma gondii belongs to the Apicomplexa phylum that caused a widespread zoonotic infection in wide range of intermediate hosts. Over one-third of the world's population are latently infected with T. gondii and carry it. The complex life cycle of T. gondii indicates the presence of a plurality of antigenic epitopes. During the recent years, continuous efforts of scientists have made precious advances to elucidate the different aspects of the cell and molecular biology of T. gondii. Despite of great progresses, the development of vaccine candidates for preventing of T. gondii infection in men and animals is still remains a challenge. The calcium-dependent protein kinases (CDPKs) belongs to the superfamily of kinases, which restricted to the apicomplexans, ciliates, and plants. It has been documented that they contribute several functions in the life cycle of T. gondii such as gliding motility, cell invasion, and egress as well as some other critical developmental processes. In current paper, we reviewed the recent progress concerning the development of CDPK-based vaccines against acute and chronic T. gondii.
Keywords: Adjuvant; Calcium-dependent protein kinase; Immunization; Toxoplasma gondii; Vaccines.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
References
-
- Nasiri V, Teymurzadeh S, Karimi G, Nasiri M. Molecular detection of Toxoplasma gondii in snakes. Exp Parasitol. 2016;169:102–106. - PubMed
-
- Dubey JP. The history of Toxoplasma gondii: the first 100 years. J Eukaryot Microbiol. 2008;55:467–475. - PubMed
-
- Foroutan M, Dalvand S, Daryani A, et al. Rolling up the pieces of a puzzle: a systematic review and meta-analysis of the prevalence of toxoplasmosis in Iran. Alex J Med. 2017 Jun 23; doi: 10.4132/10.1016/j.ajme.2017.06.003. - DOI
-
- Rostami A, Riahi SM, Fakhri Y, et al. The global seroprevalence of Toxoplasma gondii among wild boars: a systematic review and meta-analysis. Vet Parasitol. 2017;244:12–20. - PubMed
-
- Khademvatan S, Foroutan M, Hazrati-Tappeh K, et al. Toxoplasmosis in rodents: a systematic review and meta-analysis in Iran. J Infect Public Health. 2017;10:487–493. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

