A Quality Improvement Initiative to Increase Colorectal Cancer (CRC) Screening: Collaboration between a Primary Care Clinic and Research Team
- PMID: 29399669
- PMCID: PMC5792079
- DOI: 10.26420/jfammed.2017.1115
A Quality Improvement Initiative to Increase Colorectal Cancer (CRC) Screening: Collaboration between a Primary Care Clinic and Research Team
Abstract
Background: Multiple randomized controlled trials have demonstrated that mailed fecal testing programs are effective in increasing colorectal cancer screening participation. However, few healthcare organization in the US have Implemented such programs.
Methods: Stakeholders from one clinic in an integrated healthcare system in Washington State initiated collaboration with researchers with expertise in CRC screening, aiming to increase screening rates at their clinic. Age-eligible individuals who were overdue for CRC screening and had previously completed a fecal test were randomized to receive mailed fecal immunochemical test kits (FIT) at the start of the project (Early) or 6 months later (Late). Outcomes included comparing FIT completion at 6 months by randomization group, and overall CRC screening rates at 12 months. We also assessed implementation facilitators and challenges.
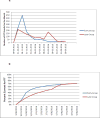
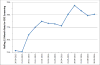
Results: Overall 2,421 FIT tests were mailed at a cost of $10,739. At 6 months, FIT completion was significantly higher among the Early compared to the Late group (62% vs.47%, p <0.001). By 12 months, after both groups had received mailings, 71% in each group had completed a FIT. The clinic's overall CRC screening rate was 75.1% at baseline and 78.0% 12 months later. Key constructs associated with successful program implementation included strong stakeholder involvement, use of evidence-based strategies, simplicity, and low cost. Challenges included lack of a plan for maintaining the program.
Discussion: Collaboration between clinic stakeholders and researchers led to a successful project that rapidly increased CRC screening rates. However, institutional normalization of the program would be required to maintain it.
Figures
Similar articles
-
First-year implementation of mailed FIT colorectal cancer screening programs in two Medicaid/Medicare health insurance plans: qualitative learnings from health plan quality improvement staff and leaders.BMC Health Serv Res. 2020 Feb 21;20(1):132. doi: 10.1186/s12913-019-4868-5. BMC Health Serv Res. 2020. PMID: 32085767 Free PMC article.
-
Mailed fecal testing and patient navigation versus usual care to improve rates of colorectal cancer screening and follow-up colonoscopy in rural Medicaid enrollees: a cluster-randomized controlled trial.Implement Sci Commun. 2022 Apr 13;3(1):42. doi: 10.1186/s43058-022-00285-3. Implement Sci Commun. 2022. PMID: 35418107 Free PMC article.
-
Challenges in Reaching Medicaid and Medicare Enrollees in a Mailed Fecal Immunochemical Test Program.J Community Health. 2020 Oct;45(5):916-921. doi: 10.1007/s10900-020-00809-9. J Community Health. 2020. PMID: 32219712
-
Improving colorectal cancer screening in rural primary care: Preliminary effectiveness and implementation of a collaborative mailed fecal immunochemical test pilot.J Rural Health. 2023 Jan;39(1):279-290. doi: 10.1111/jrh.12685. Epub 2022 Jun 15. J Rural Health. 2023. PMID: 35703582 Free PMC article.
-
Effectiveness of a mailed fecal immunochemical test outreach: a Medicare Advantage pilot study.Therap Adv Gastroenterol. 2020 Sep 9;13:1756284820945388. doi: 10.1177/1756284820945388. eCollection 2020. Therap Adv Gastroenterol. 2020. PMID: 32952612 Free PMC article.
Cited by
-
Developing Patient-Refined Messaging for a Mailed Colorectal Cancer Screening Program in a Latino-Based Community Health Center.J Am Board Fam Med. 2019 May-Jun;32(3):307-317. doi: 10.3122/jabfm.2019.03.180026. J Am Board Fam Med. 2019. PMID: 31068395 Free PMC article. Clinical Trial.
-
A cost-effectiveness analysis of a colorectal cancer screening program in safety net clinics.Prev Med. 2019 Mar;120:119-125. doi: 10.1016/j.ypmed.2019.01.014. Epub 2019 Jan 24. Prev Med. 2019. PMID: 30685318 Free PMC article.
-
First-year implementation of mailed FIT colorectal cancer screening programs in two Medicaid/Medicare health insurance plans: qualitative learnings from health plan quality improvement staff and leaders.BMC Health Serv Res. 2020 Feb 21;20(1):132. doi: 10.1186/s12913-019-4868-5. BMC Health Serv Res. 2020. PMID: 32085767 Free PMC article.
-
What's the "secret sauce"? How implementation variation affects the success of colorectal cancer screening outreach.Implement Sci Commun. 2021 Jan 11;2(1):5. doi: 10.1186/s43058-020-00104-7. Implement Sci Commun. 2021. PMID: 33431063 Free PMC article.
-
Evidence-Based Quality Improvement: a Scoping Review of the Literature.J Gen Intern Med. 2022 Dec;37(16):4257-4267. doi: 10.1007/s11606-022-07602-5. J Gen Intern Med. 2022. PMID: 36175760 Free PMC article.
References
-
- Siegel RR, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. - PubMed
-
- Vogelaar I, van Ballegooijen M, Schrag D, Boer R, Winawer SJ, Habbema JD, Zauber AG. How much can current interventions reduce colorectal cancer mortality in the U.S.? Mortality projections for scenarios of risk-factor modification, screening, and treatment. Cancer. 2006;107:1624–33. - PubMed
-
- Fielding J, Rimer B, Abraido-Lanza A, Calonge N, Clymer J, Glanz K, Goetzel R, Green L, Johnson R, Orleans C, Pronk N, Ramirez G, Dickersin K, Lawrence R, McGinnis J, Plough A, Teutsch S. Recommendations for Client- and Provider-Directed Interventions to Increase Breast, Cervical, and Colorectal Cancer Screening. Am J Prev Med. 2008;35:S21–S5. - PubMed
-
- Levy BT, Daly JM, Xu Y, Ely JW. Mailed fecal immunochemical tests plus educational materials to improve colon cancer screening rates in Iowa Research Network (IRENE) practices. J Am Board Fam Med. 2012;25:73–82. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
