Oxygen drives hepatocyte differentiation and phenotype stability in liver cell lines
- PMID: 29399736
- PMCID: PMC6039343
- DOI: 10.1007/s12079-018-0456-4
Oxygen drives hepatocyte differentiation and phenotype stability in liver cell lines
Abstract
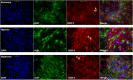
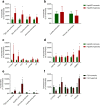
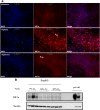
The in vitro generation of terminally differentiated hepatocytes is an unmet need. We investigated the contribution of oxygen concentration to differentiation in human liver cell lines HepaRG and C3A. HepaRG cells were cultured under hypoxia (5%O2), normoxia (21%O2) or hyperoxia (40%O2). Cultures were analysed for hepatic functions, gene transcript levels, and protein expression of albumin, hepatic transcription factor CEBPα, hepatic progenitor marker SOX9, and hypoxia inducible factor (HIF)1α. C3A cells were analysed after exposure to normoxia or hyperoxia. In hyperoxic HepaRG cultures, urea cycle activity, bile acid synthesis, CytochromeP450 3A4 (CYP3A4) activity and ammonia elimination were 165-266% increased. These effects were reproduced in C3A cells. Whole transcriptome analysis of HepaRG cells revealed that 240 (of 23.223) probes were differentially expressed under hyperoxia, with an overrepresentation of genes involved in hepatic differentiation, metabolism and extracellular signalling. Under hypoxia, CYP3A4 activity and ammonia elimination were inhibited almost completely and 5/5 tested hepatic genes and 2/3 tested hepatic transcription factor genes were downregulated. Protein expression of SOX9 and HIF1α was strongly positive in hypoxic cultures, variable in normoxic cultures and predominantly negative in hyperoxic cultures. Conversely, albumin and CEBPα expression were highest in hyperoxic cultures. HepaRG cells that were serially passaged under hypoxia maintained their capacity to differentiate under normoxia, in contrast to cells passaged under normoxia. Hyperoxia increases hepatocyte differentiation in HepaRG and C3A cells. In contrast, hypoxia maintains stem cell characteristics and inhibits hepatic differentiation of HepaRG cells, possibly through the activity of HIF1α.
Keywords: C3A; HepaRG; Hepatic progenitor cell; Hepatocyte differentiation; Oxygen; Propagation capacity.
Conflict of interest statement
RC and RH are inventors of the patent-protected HepaRG-based AMC-Bioartificial liver that is currently being commercialized through the university spin-off company Hep-Art Medical Devices B.V of which RC is unsalaried Chief Scientific Officer. RH was previously employed part-time by Hep-Art Medical Devices B.V.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

