Biomimetic Desymmetrization of a Carboxylic Acid
- PMID: 29400455
- PMCID: PMC5814305
- DOI: 10.1021/jacs.7b12185
Biomimetic Desymmetrization of a Carboxylic Acid
Abstract
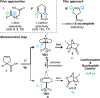
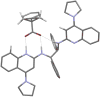
The enantioselective desymmetrization of carboxylic acids by chiral Brønsted base catalysis is reported, leading to bridged bicyclic lactones with up to 94% ee. Crystallographic analysis of a substrate-catalyst complex suggests an origin of stereocontrol, reminiscent of functional Brønsted bases in biological settings, and enabled reaction optimization. The products contain an all-carbon quaternary stereocenter and can be derivatized to functionalized cyclopentanes.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Min C, Seidel D. Chem Soc Rev. 2017:5889. - PubMed
-
- Hashimoto T, Maruoka K. J Am Chem Soc. 2007;129:10054. - PubMed
- Momiyama N, Yamamoto H. J Am Chem Soc. 2005;127:1080. - PMC - PubMed
- Hashimoto T, Maruoka K. Synthesis. 2008;2008:3703.
- Hashimoto T, Hirose M, Maruoka K. J Am Chem Soc. 2008;130:7556. - PubMed
- Hashimoto T, Nakatsu H, Takiguchi Y, Maruoka K. J Am Chem Soc. 2013;135:16010. - PubMed
-
- Zhang J, Lin SX, Cheng DJ, Liu XY, Tan B. J Am Chem Soc. 2015;137:14039. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

