Randomized, controlled trial of TNF-α antagonist in CTL-mediated severe cutaneous adverse reactions
- PMID: 29400697
- PMCID: PMC5824923
- DOI: 10.1172/JCI93349
Randomized, controlled trial of TNF-α antagonist in CTL-mediated severe cutaneous adverse reactions
Abstract
Background: Cytotoxic T lymphocyte-mediated (CTL-mediated) severe cutaneous adverse reactions (SCARs), including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), are rare but life-threatening adverse reactions commonly induced by drugs. Although high levels of CTL-associated cytokines, chemokines, or cytotoxic proteins, including TNF-α and granulysin, were observed in SJS-TEN patients in recent studies, the optimal treatment for these diseases remains controversial. We aimed to evaluate the efficacy, safety, and therapeutic mechanism of a TNF-α antagonist in CTL-mediated SCARs.
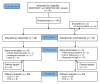
Methods: We enrolled 96 patients with SJS-TEN in a randomized trial to compare the effects of the TNF-α antagonist etanercept versus traditional corticosteroids.
Results: Etanercept improved clinical outcomes in patients with SJS-TEN. Etanercept decreased the SCORTEN-based predicted mortality rate (predicted and observed rates, 17.7% and 8.3%, respectively). Compared with corticosteroids, etanercept further reduced the skin-healing time in moderate-to-severe SJS-TEN patients (median time for skin healing was 14 and 19 days for etanercept and corticosteroids, respectively; P = 0.010), with a lower incidence of gastrointestinal hemorrhage in all SJS-TEN patients (2.6% for etanercept and 18.2% for corticosteroids; P = 0.03). In the therapeutic mechanism study, etanercept decreased the TNF-α and granulysin secretions in blister fluids and plasma (45.7%-62.5% decrease after treatment; all P < 0.05) and increased the Treg population (2-fold percentage increase after treatment; P = 0.002), which was related to mortality in severe SJS-TEN.
Conclusions: The anti-TNF-α biologic agent etanercept serves as an effective alternative for the treatment of CTL-mediated SCARs.
Trial registration: ClinicalTrials.gov NCT01276314.
Funding: Ministry of Science and Technology of Taiwan.
Keywords: Allergy; Clinical Trials; Immunology; Immunotherapy.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

