Clonally expanded γδ T cells protect against Staphylococcus aureus skin reinfection
- PMID: 29400698
- PMCID: PMC5824877
- DOI: 10.1172/JCI96481
Clonally expanded γδ T cells protect against Staphylococcus aureus skin reinfection
Abstract
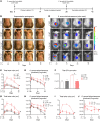
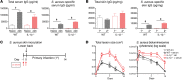
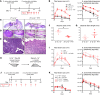
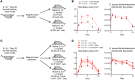
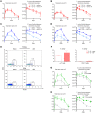
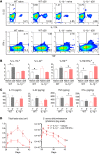
The mechanisms that mediate durable protection against Staphylococcus aureus skin reinfections are unclear, as recurrences are common despite high antibody titers and memory T cells. Here, we developed a mouse model of S. aureus skin reinfection to investigate protective memory responses. In contrast with WT mice, IL-1β-deficient mice exhibited poor neutrophil recruitment and bacterial clearance during primary infection that was rescued during secondary S. aureus challenge. The γδ T cells from skin-draining LNs utilized compensatory T cell-intrinsic TLR2/MyD88 signaling to mediate rescue by trafficking and producing TNF and IFN-γ, which restored neutrophil recruitment and promoted bacterial clearance. RNA-sequencing (RNA-seq) of the LNs revealed a clonotypic S. aureus-induced γδ T cell expansion with a complementarity-determining region 3 (CDR3) aa sequence identical to that of invariant Vγ5+ dendritic epidermal T cells. However, this T cell receptor γ (TRG) aa sequence of the dominant CDR3 sequence was generated from multiple gene rearrangements of TRGV5 and TRGV6, indicating clonotypic expansion. TNF- and IFN-γ-producing γδ T cells were also expanded in peripheral blood of IRAK4-deficient humans no longer predisposed to S. aureus skin infections. Thus, clonally expanded γδ T cells represent a mechanism for long-lasting immunity against recurrent S. aureus skin infections.
Keywords: Adaptive immunity; Bacterial infections; Immunology; Infectious disease; Skin.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

