Ex vivo expansion of alveolar macrophages with Mycobacterium tuberculosis from the resected lungs of patients with pulmonary tuberculosis
- PMID: 29401466
- PMCID: PMC5798839
- DOI: 10.1371/journal.pone.0191918
Ex vivo expansion of alveolar macrophages with Mycobacterium tuberculosis from the resected lungs of patients with pulmonary tuberculosis
Abstract
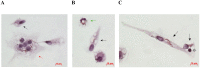
Tuberculosis (TB), with the Mycobacterium tuberculosis (Mtb) as the causative agent, remains to be a serious world health problem. Traditional methods used for the study of Mtb in the lungs of TB patients do not provide information about the number and functional status of Mtb, especially if Mtb are located in alveolar macrophages. We have developed a technique to produce ex vivo cultures of cells from different parts of lung tissues surgically removed from patients with pulmonary TB and compared data on the number of cells with Mtb inferred by the proposed technique to the results of bacteriological and histological analyses used for examination of the resected lungs. The ex vivo cultures of cells obtained from the resected lungs of all patients were largely composed of CD14-positive alveolar macrophages, foamy or not, with or without Mtb. Lymphocytes, fibroblasts, neutrophils, and multinucleate Langhans giant cells were also observed. We found alveolar macrophages with Mtb in the ex vivo cultures of cells from the resected lungs of even those TB patients, whose sputum smears and lung tissues did not contain acid-fast Mtb or reveal growing Mtb colonies on dense medium. The detection of alveolar macrophages with Mtb in ex vivo culture as soon as 16-18 h after isolation of cells from the resected lungs of all TB patients suggests that the technique proposed for assessing the level of infection in alveolar macrophages of TB patients has higher sensitivity than do prolonged bacteriological or pathomorphological methods. The proposed technique allowed us to rapidly (in two days after surgery) determine the level of infection with Mtb in the cells of the resected lungs of TB patients and, by the presence or absence of Mtb colonies, including those with cording morphology, the functional status of the TB agent at the time of surgery.
Conflict of interest statement
Figures




Similar articles
-
Analysis of Mycobacterium tuberculosis Uptake by Alveolar Macrophages after Ex vivo Expansion Indicates Processing Host Cells with Pathogen Actually from Lung Tissue of Patients with Pulmonary Tuberculosis.Int J Mycobacteriol. 2020 Apr-Jun;9(2):176-184. doi: 10.4103/ijmy.ijmy_39_20. Int J Mycobacteriol. 2020. PMID: 32474540
-
Mycobacterium tuberculosis cording in alveolar macrophages of patients with pulmonary tuberculosis is likely associated with increased mycobacterial virulence.Tuberculosis (Edinb). 2018 Sep;112:1-10. doi: 10.1016/j.tube.2018.07.001. Epub 2018 Jul 3. Tuberculosis (Edinb). 2018. PMID: 30205961
-
Mycobacterium tuberculosis with different virulence reside within intact phagosomes and inhibit phagolysosomal biogenesis in alveolar macrophages of patients with pulmonary tuberculosis.Tuberculosis (Edinb). 2019 Jan;114:77-90. doi: 10.1016/j.tube.2018.12.002. Epub 2018 Dec 6. Tuberculosis (Edinb). 2019. PMID: 30711161
-
Mycobacterium tuberculosis Infection-Driven Foamy Macrophages and Their Implications in Tuberculosis Control as Targets for Host-Directed Therapy.Front Immunol. 2020 May 12;11:910. doi: 10.3389/fimmu.2020.00910. eCollection 2020. Front Immunol. 2020. PMID: 32477367 Free PMC article. Review.
-
Mycobacterium tuberculosis-macrophage interaction: Molecular updates.Front Cell Infect Microbiol. 2023 Mar 3;13:1062963. doi: 10.3389/fcimb.2023.1062963. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 36936766 Free PMC article. Review.
Cited by
-
Drug-Tolerant Mycobacterium tuberculosis Adopt Different Survival Strategies in Alveolar Macrophages of Patients with Pulmonary Tuberculosis.Int J Mol Sci. 2023 Oct 6;24(19):14942. doi: 10.3390/ijms241914942. Int J Mol Sci. 2023. PMID: 37834390 Free PMC article.
-
Alveolar macrophages in tissue homeostasis, inflammation, and infection: evolving concepts of therapeutic targeting.J Clin Invest. 2023 Oct 2;133(19):e170501. doi: 10.1172/JCI170501. J Clin Invest. 2023. PMID: 37781922 Free PMC article. Review.
-
Mycobacterium tuberculosis Load in Host Cells and the Antibacterial Activity of Alveolar Macrophages Are Linked and Differentially Regulated in Various Lung Lesions of Patients with Pulmonary Tuberculosis.Int J Mol Sci. 2021 Mar 26;22(7):3452. doi: 10.3390/ijms22073452. Int J Mol Sci. 2021. PMID: 33810600 Free PMC article.
-
Development of Ex Vivo Analysis for Examining Cell Composition, Immunological Landscape, Tumor and Immune Related Markers in Non-Small-Cell Lung Cancer.Cancers (Basel). 2024 Aug 20;16(16):2886. doi: 10.3390/cancers16162886. Cancers (Basel). 2024. PMID: 39199657 Free PMC article.
-
Mycobacterium tuberculosis Metabolism.Microbiol Spectr. 2019 Jul;7(4):10.1128/microbiolspec.gpp3-0067-2019. doi: 10.1128/microbiolspec.GPP3-0067-2019. Microbiol Spectr. 2019. PMID: 31350832 Free PMC article. Review.
References
-
- World Health Organization. Global Tuberculosis Report 2016.
-
- Lawn SD, Zumla A. Tuberculosis. Lancet. 2011;378(9785):57–72. doi: 10.1016/S0140-6736(10)62173-3 - DOI - PubMed
-
- Cheallaigh CN, de Castro CP, Coleman MM, Hope JC, Harris J. Macrophages and tuberculosis In: Takahashi R, Kai H, editors. Handbook of macrophages. New York: Nova Science Publishers; 2012. pp. 1–41.
-
- Sasindran SJ, Torrelles JB. Mycobacterium tuberculosis infection and inflammation: what is beneficial for the host and for the bacterium? Front Microbiol. 2011;2:2 doi: 10.3389/fmicb.2011.00002 - DOI - PMC - PubMed
-
- Hmama Z, Pena-Diaz S, Joseph S, Av-Gay Y. Immunoevasion and immunosuppression of the macrophage by Mycobacterium tuberculosis. Immunol Rev. 2015;264(1):220–32. doi: 10.1111/imr.12268 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

