Hexons from adenovirus serotypes 5 and 48 differentially protect adenovirus vectors from neutralization by mouse and human serum
- PMID: 29401488
- PMCID: PMC5798830
- DOI: 10.1371/journal.pone.0192353
Hexons from adenovirus serotypes 5 and 48 differentially protect adenovirus vectors from neutralization by mouse and human serum
Abstract
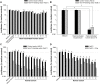
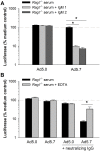
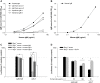
Adenovirus vectors are widely used in gene therapy clinical trials, and preclinical studies with these vectors are often conducted in mice. It is therefore critical to understand whether mouse studies adequately predict the behavior of adenovirus vectors in humans. The most commonly-used adenovirus vectors are derived from adenovirus serotype 5 (Ad5). The Ad5 hexon protein can bind coagulation factor X (FX), and binding of FX has a major impact on vector interactions with other blood proteins. In mouse serum, FX protects Ad5 vectors from neutralization by natural antibodies and complement. In the current study, we similarly find that human FX inhibits neutralization of Ad5 vectors by human serum, and this finding is consistent among individual human sera. We show that human IgM and human IgG can each induce complement-mediated neutralization when Ad5 vectors are not protected by FX. Although mouse and human serum had similar effects on Ad5 vectors, we found that this was not true for a chimeric Ad5 vector that incorporated hexon regions from adenovirus serotype 48. Interestingly, this hexon-chimeric vector was neutralized by human serum, but not by mouse serum. These findings indicate that studies in mouse serum accurately predict the behavior of Ad5 vectors in human serum, but mouse serum is not an accurate model system for all adenovirus vectors.
Conflict of interest statement
Figures


Similar articles
-
Binding of adenovirus species C hexon to prothrombin and the influence of hexon on vector properties in vitro and in vivo.PLoS Pathog. 2022 Sep 26;18(9):e1010859. doi: 10.1371/journal.ppat.1010859. eCollection 2022 Sep. PLoS Pathog. 2022. PMID: 36156097 Free PMC article.
-
Substitution of blood coagulation factor X-binding to Ad5 by position-specific PEGylation: Preventing vector clearance and preserving infectivity.J Control Release. 2016 Aug 10;235:379-392. doi: 10.1016/j.jconrel.2016.06.022. Epub 2016 Jun 11. J Control Release. 2016. PMID: 27302248
-
Modification of Ad5 hexon hypervariable regions circumvents pre-existing Ad5 neutralizing antibodies and induces protective immune responses.PLoS One. 2012;7(4):e33920. doi: 10.1371/journal.pone.0033920. Epub 2012 Apr 5. PLoS One. 2012. PMID: 22496772 Free PMC article.
-
Development and evaluation of a novel gene delivery vehicle composed of adenovirus serotype 35.Biol Pharm Bull. 2008 Oct;31(10):1819-25. doi: 10.1248/bpb.31.1819. Biol Pharm Bull. 2008. PMID: 18827334 Review.
-
The influence of innate and pre-existing immunity on adenovirus therapy.J Cell Biochem. 2009 Nov 1;108(4):778-90. doi: 10.1002/jcb.22328. J Cell Biochem. 2009. PMID: 19711370 Free PMC article. Review.
Cited by
-
Adenoviruses in medicine: innocuous pathogen, predator, or partner.Trends Mol Med. 2023 Jan;29(1):4-19. doi: 10.1016/j.molmed.2022.10.001. Epub 2022 Nov 4. Trends Mol Med. 2023. PMID: 36336610 Free PMC article. Review.
-
Adenovirus Type 6: Subtle Structural Distinctions from Adenovirus Type 5 Result in Essential Differences in Properties and Perspectives for Gene Therapy.Pharmaceutics. 2021 Oct 8;13(10):1641. doi: 10.3390/pharmaceutics13101641. Pharmaceutics. 2021. PMID: 34683934 Free PMC article. Review.
-
Binding of adenovirus species C hexon to prothrombin and the influence of hexon on vector properties in vitro and in vivo.PLoS Pathog. 2022 Sep 26;18(9):e1010859. doi: 10.1371/journal.ppat.1010859. eCollection 2022 Sep. PLoS Pathog. 2022. PMID: 36156097 Free PMC article.
-
Human Adenovirus Serotype 5 Is Sensitive to IgM-Independent Neutralization In Vitro and In Vivo.Viruses. 2019 Jul 5;11(7):616. doi: 10.3390/v11070616. Viruses. 2019. PMID: 31284434 Free PMC article.
-
Antibodies against adenovirus fiber and penton base proteins inhibit adenovirus vector-mediated transduction in the liver following systemic administration.Sci Rep. 2018 Aug 17;8(1):12315. doi: 10.1038/s41598-018-30947-z. Sci Rep. 2018. PMID: 30120324 Free PMC article.
References
-
- The Journal of Gene Medicine Gene Therapy Clinical Trials Worldwide 2017. http://www.wiley.com/legacy/wileychi/genmed/clinical/.
-
- Harmon AW, Byrnes AP. Adenovirus vector toxicity In: Brunetti-Pierri N, editor. Safety and efficacy of gene-based therapeutics for inherited disorders: Springer International Publishing; 2017. p. 37–60.
-
- Di Paolo NC, van Rooijen N, Shayakhmetov DM. Redundant and synergistic mechanisms control the sequestration of blood-born adenovirus in the liver. Mol Ther. 2009;17(4):675–84. doi: 10.1038/mt.2008.307 - DOI - PMC - PubMed
-
- Lopez-Gordo E, Denby L, Nicklin SA, Baker AH. The importance of coagulation factors binding to adenovirus: historical perspectives and implications for gene delivery. Expert Opin Drug Deliv. 2014:1–19. - PubMed
-
- Waddington SN, McVey JH, Bhella D, Parker AL, Barker K, Atoda H, et al. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008;132(3):397–409. doi: 10.1016/j.cell.2008.01.016 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

