Neutrophil predominance in bronchoalveolar lavage fluid is associated with disease severity and progression of HRCT findings in pulmonary Mycobacterium avium infection
- PMID: 29401501
- PMCID: PMC5798761
- DOI: 10.1371/journal.pone.0190189
Neutrophil predominance in bronchoalveolar lavage fluid is associated with disease severity and progression of HRCT findings in pulmonary Mycobacterium avium infection
Abstract
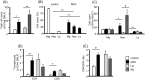
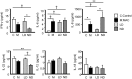
Pulmonary Mycobacterium avium complex (MAC) infection is increasing in prevalence worldwide even in immunocompetent individuals. Despite its variable clinical course, the clinical and immunological factors associated with radiographical severity and progression are not largely unknown. We aimed to study the association between the inflammatory cell and cytokine profiles at the local infected site, and the radiological severity and/or progression of pulmonary MAC infection. In this retrospective cohort study, 22 healthy subjects and 37 consecutive patients who were diagnosed as having pulmonary MAC infection by positive cultures of bronchoalveolar lavage (BAL) fluids were enrolled. The 37 patients were divided into 2 groups based on the predominant BAL inflammatory cell type: the lymphocyte-dominant (LD) group and neutrophil-dominant (ND) groups. The high-resolution computed tomography score in both the lavaged segment and whole lung and cytokines profiles were compared between the 2 groups. The clinical course after the BAL procedure was also compared between the 2 groups. Both the segment and whole lung scores in the ND group were significantly higher than the LD group (P < 0.001). Levels of IL-8 in the BAL fluids were significantly higher in the ND group compared to the LD group (P = 0.01). In contrast, levels of IL-22 were significantly lower in the ND group compared to the LD group (P < 0.001). The prevalence of patients who showed deterioration of the disease was significantly higher in the ND group (83.3%) than the LD group (12.5%) (P < 0.01). Neutrophil-predominant inflammatory response at the infected site is associated with the radiographical severity and progression of pulmonary MAC infection.
Conflict of interest statement
Figures
Similar articles
-
Clinical investigation of pulmonary Mycobacterium avium complex infection in human T lymphotrophic virus type I carriers.Thorax. 2000 May;55(5):388-92. doi: 10.1136/thorax.55.5.388. Thorax. 2000. PMID: 10770820 Free PMC article.
-
Lack of T-lymphocytosis and poor interferon gamma production in BAL fluid from HIV-negative immunocompetent patients with pulmonary non-tuberculous mycobacteriosis.Scand J Infect Dis. 1998;30(4):339-43. doi: 10.1080/00365549850160602. Scand J Infect Dis. 1998. PMID: 9817511
-
Mycobacterium avium-intracellulare pulmonary infection in patients without known predisposing lung disease.Lung. 1998;176(6):381-91. doi: 10.1007/pl00007620. Lung. 1998. PMID: 9780296
-
High-resolution computed tomography appearance of pulmonary Mycobacterium avium complex infection after exposure to hot tub: case of hot-tub lung.J Thorac Imaging. 2003 Jan;18(1):48-52. doi: 10.1097/00005382-200301000-00008. J Thorac Imaging. 2003. PMID: 12544748 Review.
-
[Hot tub lung].Nihon Naika Gakkai Zasshi. 2006 Jun 10;95(6):1013-8. doi: 10.2169/naika.95.1013. Nihon Naika Gakkai Zasshi. 2006. PMID: 16846048 Review. Japanese. No abstract available.
Cited by
-
A two-sample mendelian randomization study of non-tuberculous mycobacteria infection and lung cancer.J Thorac Dis. 2024 Dec 31;16(12):8472-8481. doi: 10.21037/jtd-24-1268. Epub 2024 Dec 12. J Thorac Dis. 2024. PMID: 39831261 Free PMC article.
-
Antimicrobial Activity of Neutrophils Against Mycobacteria.Front Immunol. 2021 Dec 23;12:782495. doi: 10.3389/fimmu.2021.782495. eCollection 2021. Front Immunol. 2021. PMID: 35003097 Free PMC article. Review.
-
Bronchoalveolar lavage as a diagnostic procedure: a review of known cellular and molecular findings in various lung diseases.J Thorac Dis. 2020 Sep;12(9):4991-5019. doi: 10.21037/jtd-20-651. J Thorac Dis. 2020. PMID: 33145073 Free PMC article. Review.
-
IL-22: An Underestimated Player in Natural Resistance to Tuberculosis?Front Immunol. 2018 Sep 25;9:2209. doi: 10.3389/fimmu.2018.02209. eCollection 2018. Front Immunol. 2018. PMID: 30319650 Free PMC article. Review.
-
The factors associated with mortality and progressive disease of nontuberculous mycobacterial lung disease: a systematic review and meta-analysis.Sci Rep. 2023 May 5;13(1):7348. doi: 10.1038/s41598-023-34576-z. Sci Rep. 2023. PMID: 37147519 Free PMC article.
References
-
- Donohue MJ, Wymer L. Increasing Prevalence Rate of Nontuberculous Mycobacteria Infections in Five States, 2008–2013. Ann Am Thorac Soc. 2016;13(12): 2143–50. doi: 10.1513/AnnalsATS.201605-353OC - DOI - PubMed
-
- Ide S, Nakamura S, Yamamoto Y, Kohno Y, Fukuda Y, Ikeda H, et al. Epidemiology and clinical features of pulmonary nontuberculous mycobacteriosis in Nagasaki, Japan. PLoS One. 2015;28(5): e0128304 doi: 10.1371/journal.pone.0128304 - DOI - PMC - PubMed
-
- Wu J, Zhang Y, Li J, Lin S, Wang L, Jiang Y, et al. Increase in nontuberculous mycobacteria isolated in Shanghai, China: results from a population-based study. PLoS One. 2014;16(10): e109736 doi: 10.1371/journal.pone.0109736 - DOI - PMC - PubMed
-
- Cassidy PM, Hedberg K, Saulson A, McNelly E, Winthrop KL. Nontuberculous mycobacterial disease prevalence and risk factors: a changing epidemiology. Clin Infect Dis. 2009;49(12): e124–9. doi: 10.1086/648443 - DOI - PubMed
-
- Morimoto K, Iwai K, Uchimura K, Okumura M, Yoshiyama T, Yoshimori K, et al. A steady increase in nontuberculous mycobacteriosis mortality and estimated prevalence in Japan. Ann Am Thorac Soc. 2014;11(1): 1–8. doi: 10.1513/AnnalsATS.201303-067OC - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

