Dysregulated bile acid synthesis and dysbiosis are implicated in Western diet-induced systemic inflammation, microglial activation, and reduced neuroplasticity
- PMID: 29401580
- PMCID: PMC5901391
- DOI: 10.1096/fj.201700984RR
Dysregulated bile acid synthesis and dysbiosis are implicated in Western diet-induced systemic inflammation, microglial activation, and reduced neuroplasticity
Abstract
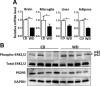
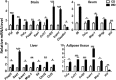
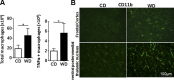
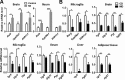
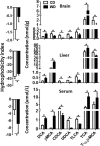
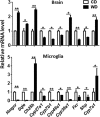
The goal of this study was to identify the intrinsic links that explain the effect of a Western diet (WD) on cognitive dysfunction. Specific pathogen-free, wild-type mice were fed either a control diet (CD) or a high-fat, high-sucrose WD after weaning and were euthanized at 10 mo of age to study the pathways that affect cognitive health. The results showed that long-term WD intake reduced hippocampal synaptic plasticity and the level of brain-derived neurotrophic factor mRNA in the brain and isolated microglia. A WD also activated ERK1/2 and reduced postsynaptic density-95 in the brain, suggesting postsynaptic damage. Moreover, WD-fed mice had increased inflammatory signaling in the brain, ileum, liver, adipose tissue, and spleen, which was accompanied by microglia activation. In the brain, as well as in the digestive tract, a WD reduced signaling regulated by retinoic acid and bile acids (BAs), whose receptors form heterodimers to control metabolism and inflammation. Furthermore, a WD intake caused dysbiosis and dysregulated BA synthesis with reduced endogenous ligands for BA receptors, i.e., farnesoid X receptor and G-protein-coupled bile acid receptor in the liver and brain. Together, dysregulated BA synthesis and dysbiosis were accompanied by systemic inflammation, microglial activation, and reduced neuroplasticity induced by WD.-Jena, P. K., Sheng, L., Di Lucente, J., Jin, L.-W., Maezawa, I., Wan, Y.-J. Y. Dysregulated bile acid synthesis and dysbiosis are implicated in Western diet-induced systemic inflammation, microglial activation, and reduced neuroplasticity.
Keywords: Alzheimer’s disease; FXR; TGR5; cognition; gut microbiota.
Conflict of interest statement
The authors thank Dr. Betty P. Guo for extensive review and editing of this manuscript, and Niki Taylor DeGeorge for editing (both from the University of California, Davis). This study was supported by U.S. National Institutes of Health (NIH) National Cancer Institute Grant U01CA179582, and NIH National Institute on Aging Grants P30AG010129 and R01AG043788. The authors declare no conflicts of interest.
Figures



References
-
- Friedland R. P. (2015) Mechanisms of molecular mimicry involving the microbiota in neurodegeneration. J. Alzheimers Dis. 45, 349–362 - PubMed
-
- Pistollato F., Sumalla Cano S., Elio I., Masias Vergara M., Giampieri F., Battino M. (2016) Role of gut microbiota and nutrients in amyloid formation and pathogenesis of Alzheimer disease. Nutr. Rev. 74, 624–634 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

