The Taste of Commercially Available Clarithromycin Oral Pharmaceutical Suspensions in the Palestinian Market: Electronic Tongue and In Vivo Evaluation
- PMID: 29401675
- PMCID: PMC5855094
- DOI: 10.3390/s18020454
The Taste of Commercially Available Clarithromycin Oral Pharmaceutical Suspensions in the Palestinian Market: Electronic Tongue and In Vivo Evaluation
Abstract
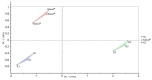
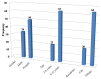
Background: The taste of oral liquid dosage forms is a crucial factor that impacts paediatric patient compliance. The electronic tongue (ET) is an emerging tool that could be useful in taste assessment in order to minimize the involvement of humans in such evaluations. Purpose: The aim of this study is to evaluate the taste of commercially available clarithromycin (CM) oral pharmaceutical suspensions in the Palestinian market. Method: Commercially available CM suspensions (the brand Klacid® and two generic K1 and K2) were assayed using the high performance liquid chromatography (HPLC) method. Then, the taste of these products was assessed using alpha-astree ET. In addition, an in vivo taste assessment was conducted on paediatric patients by a hedonic panel test. Moreover, volunteering community pharmacists were asked to rank the taste of these three products according to their experience from the best to the worst. Results: All suspension products had a CM concentration not less than 98% of the label amount. The ET results coupled with the principal component analysis (PCA) showed a very clear discrimination of the samples with different distances between groups (p-values < 0.001). Suspensions were in the following order in terms of taste: Klacid® > K1 > K2. Moreover, The pattern discrimination index between (K1 and Klacid®), (K1 and K2) and (Klacid® and K2) were 8.81%, 65.75%, and71.94%, respectively which suggests that K1 and Klacid® are the most similar preparations in terms of taste. Interestingly, these results were in excellent agreement with the pharmacist ranking and patient acceptance test. Conclusions: The evaluated preparations showed significantly different taste within the order of Klacid® > K1 > K2, as suggested by both the ET and in vivo results. Moreover, our results confirm the capability of alpha-astree ET in the taste assessment of oral suspensions and in predicting volunteer responses, which highlights its beneficial use as an in vitro taste assessment tool and as an alternative to human-based taste evaluations.
Keywords: alpha-astree; clarithromycin; electronic tongue; pediatric; taste.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Draft Guideline on Pharmaceutical Development of Medicines for Paediatric Use. [(accessed on 24 December 2017)];2011 EMA/CHMP/QWP/180157/2011 Committee for Medicinal Products for Human Use (CHMP) Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin....
-
- Peng F., Wang W., Luo W., Liu Y., Li H. Application of near-infrared spectroscopy for monitoring the formulation process of low-dose tablets. Anal. Methods. 2014;6:1905–1913. doi: 10.1039/C3AY41841C. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

