Elevated Polyamines in Saliva of Pancreatic Cancer
- PMID: 29401744
- PMCID: PMC5836075
- DOI: 10.3390/cancers10020043
Elevated Polyamines in Saliva of Pancreatic Cancer
Abstract
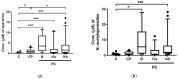
Detection of pancreatic cancer (PC) at a resectable stage is still difficult because of the lack of accurate detection tests. The development of accurate biomarkers in low or non-invasive biofluids is essential to enable frequent tests, which would help increase the opportunity of PC detection in early stages. Polyamines have been reported as possible biomarkers in urine and saliva samples in various cancers. Here, we analyzed salivary metabolites, including polyamines, using capillary electrophoresis-mass spectrometry. Salivary samples were collected from patients with PC (n = 39), those with chronic pancreatitis (CP, n = 14), and controls (C, n = 26). Polyamines, such as spermine, N₁-acetylspermidine, and N₁-acetylspermine, showed a significant difference between patients with PC and those with C, and the combination of four metabolites including N₁-acetylspermidine showed high accuracy in discriminating PC from the other two groups. These data show the potential of saliva as a source for tests screening for PC.
Keywords: metabolomics; pancreatic cancer; polyamines; saliva.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Canto M.I., Hruban R.H., Fishman E.K., Kamel I.R., Schulick R., Zhang Z., Topazian M., Takahashi N., Fletcher J., Petersen G., et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology. 2012;142:796–804. doi: 10.1053/j.gastro.2012.01.005. quiz e714-795. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

