Featured Article: Immunomodulatory effect of hemozoin on pneumocyte apoptosis via CARD9 pathway, a possibly retarding pulmonary resolution
- PMID: 29402133
- PMCID: PMC5882027
- DOI: 10.1177/1535370218757458
Featured Article: Immunomodulatory effect of hemozoin on pneumocyte apoptosis via CARD9 pathway, a possibly retarding pulmonary resolution
Abstract
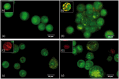
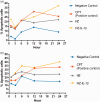
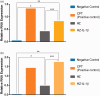
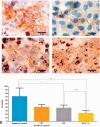
Plasmodium falciparum, the most virulent malaria parasite species, causes severe symptoms especially acute lung injury (ALI), of which characterized by alveolar epithelium and endothelium destruction and accelerated to blood-gas-barrier breakdown. Parasitized erythrocytes, endothelial cells, monocytes, and cytokines are all involved in this mechanism, but hemozoin (HZ), the parasitic waste from heme detoxification, also mainly contributes. In addition, it is not clear why type II pneumocyte proliferation, alveolar restorative stage, is rare in malaria-associated ALI. To address this, in vitro culture of A549 cells with Plasmodium HZ or with interleukin (IL)-1β triggered by HZ and monocytes (HZ-IL-1β) was conducted to determine their alveolar apoptotic effect using ethidium bromide/acridine orange staining, annexin-V-FITC/propidium iodide staining, and electron mircroscopic study. Caspase recruitment domain-containing protein 9 ( CARD9), the apoptotic regulator gene, and IL-1β were quantified by reverse-transcriptase PCR. Junctional cellular defects were characterized by immunohistochemical staining of E-cadherin. The results revealed that cellular apoptosis and CARD9 expression levels were extremely high 24 h after induction by HZ-IL-1β when compared to the HZ- and non-treated groups. E-cadherin was markedly down-regulated by HZ-IL-1β and HZ treatments. CARD9 expression was positively correlated with IL-1β expression and the number of apoptotic cells. Interestingly, the localization of HZ in the vesicular surfactant of apoptotic pneumocyte was also identified and submitted to be a cause of alveolar resolution abnormality. Thus, HZ triggers monocytes to produce IL-1β and induces pneumocyte type II apoptosis through CARD9 pathway in association with down-regulated E-cadherin, which probably impairs alveolar resolution in malaria-associated ALI. Impact statement The present work shows the physical and immunomodulatory properties of hemozoin on the induction of pneumocyte apoptosis in relation to IL-1β production through the CARD9 pathway. This occurrence may be a possible pathway for the retardation of lung resolution leading to blood-gas-barrier breakdown. Our findings lead to the understanding of the host-parasite relationship focusing on the dysfunction in ALI induced by HZ, a possible pathway of the recovering lung epithelial retardation in malaria-associated ARDS.
Keywords: Acute lung injury; CARD9; apoptosis; hemozoin; interleukin-1β.
Figures







References
-
- Sein KK, Maeno Y, Thuc HV, Anh TK, Aikawa M. Differential sequestration of parasitized erythrocytes in the cerebrum and cerebellum in human cerebral malaria. Am J Trop Med Hyg 1993; 48:504–11 - PubMed
-
- Nguansangiam S, Day NP, Hien TT, Mai NT, Chaisri U, Riganti M, Dondorp AM, Lee SJ, Phu NH, Turner GD, White NJ, Ferguson DJ, Pongponratn E. A quantitative ultrastructural study of renal pathology in fatal Plasmodium falciparum malaria. Trop Med Int Health 2007; 12:1037–50 - PubMed
-
- Taylor WR, Hanson J, Turner GD, White NJ, Dondorp AM. Respiratory manifestations of malaria. Chest 2012; 142:492–505 - PubMed
-
- Mohan A, Sharma SK, Bollineni S. Acute lung injury and acute respiratory distress syndrome in malaria. J Vector Borne Dis 2008; 45:179–93 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

