Trial protocol: a parallel group, individually randomized clinical trial to evaluate the effect of a mobile phone application to improve sexual health among youth in Stockholm County
- PMID: 29402241
- PMCID: PMC5799911
- DOI: 10.1186/s12889-018-5110-9
Trial protocol: a parallel group, individually randomized clinical trial to evaluate the effect of a mobile phone application to improve sexual health among youth in Stockholm County
Abstract
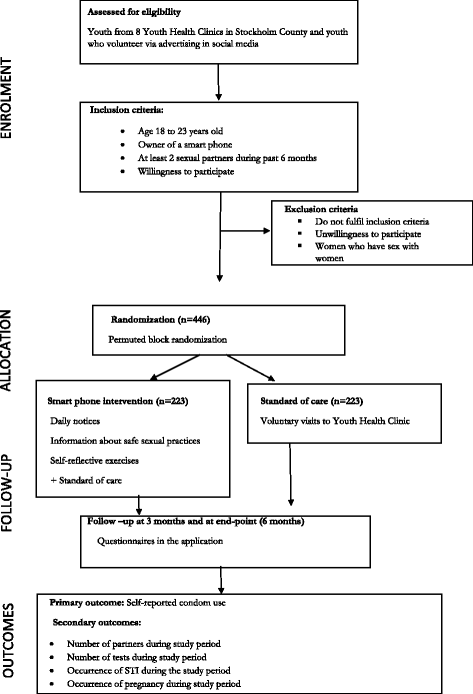
Background: Genital Chlamydia trachomatis infection is a major public health problem worldwide affecting mostly youth. Sweden introduced an opportunistic screening approach in 1982 accompanied by treatment, partner notification and case reporting. After an initial decline in infection rate till the mid-90s, the number of reported cases has increased over the last two decades and has now stabilized at a high level of 37,000 reported cases in Sweden per year (85% of cases in youth). Sexual risk-taking among youth is also reported to have significantly increased over the last 20 years. Mobile health (mHealth) interventions could be particularly suitable for youth and sexual health promotion as the intervention is delivered in a familiar and discrete way to a tech savvy at-risk population. This paper presents a protocol for a randomized trial to study the effect of an interactive mHealth application (app) on condom use among the youth of Stockholm.
Methods: 446 youth resident in Stockholm, will be recruited in this two arm parallel group individually randomized trial. Recruitment will be from Youth Health Clinics or via the trial website. Participants will be randomized to receive either the intervention (which comprises an interactive app on safe sexual health that will be installed on their smart phones) or a control group (standard of care). Youth will be followed up for 6 months, with questionnaire responses submitted periodically via the app. Self-reported condom use over 6 months will be the primary outcome. Secondary outcomes will include presence of an infection, Chlamydia tests during the study period and proxy markers of safe sex. Analysis is by intention to treat.
Discussion: This trial exploits the high mobile phone usage among youth to provide a phone app intervention in the area of sexual health. If successful, the results will have implications for health service delivery and health promotion among the youth. From a methodological perspective, this trial is expected to provide information on the strength and challenges of implementing a partially app (internet) based trial in this context.
Trial registration: ISRCTN 13212899, date of registration June 22, 2017.
Keywords: Chlamydia trachomatis; Pragmatic randomized controlled trial; Youth; mHealth; smart-phone application.
Conflict of interest statement
Ethics approval and consent to participate
Written informed consent will be obtained from each participant. Participation is voluntary, confidentiality is assured and each participant will be informed that they can withdraw from the study at any time. The research protocol was approved by the Stockholm Regional Ethical board (reference number: 2017/651–31/4).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Similar articles
-
Development of a Mobile Phone App to Promote Safe Sex Practice Among Youth in Stockholm, Sweden: Qualitative Study.JMIR Form Res. 2020 Jan 28;4(1):e12917. doi: 10.2196/12917. JMIR Form Res. 2020. PMID: 32012038 Free PMC article.
-
The MOSEXY trial: mobile phone intervention for sexual health in youth-a pragmatic randomised controlled trial to evaluate the effect of a smartphone application on sexual health in youth in Stockholm, Sweden.Sex Transm Infect. 2021 Mar;97(2):141-146. doi: 10.1136/sextrans-2019-054027. Epub 2019 Oct 18. Sex Transm Infect. 2021. PMID: 31628248 Free PMC article. Clinical Trial.
-
Study protocol: using a mobile phone-based application to increase awareness and uptake of sexual and reproductive health services among the youth in Uganda. A randomized controlled trial.Reprod Health. 2018 Dec 22;15(1):216. doi: 10.1186/s12978-018-0642-0. Reprod Health. 2018. PMID: 30577872 Free PMC article.
-
Meet us on the phone: mobile phone programs for adolescent sexual and reproductive health in low-to-middle income countries.Reprod Health. 2017 Jan 17;14(1):11. doi: 10.1186/s12978-016-0276-z. Reprod Health. 2017. PMID: 28095855 Free PMC article. Review.
-
Crowdsourcing to Improve HIV and Sexual Health Outcomes: a Scoping Review.Curr HIV/AIDS Rep. 2019 Aug;16(4):270-278. doi: 10.1007/s11904-019-00448-3. Curr HIV/AIDS Rep. 2019. PMID: 31155691 Free PMC article.
Cited by
-
Methods for Analyzing the Contents of Social Media for Health Care: Scoping Review.J Med Internet Res. 2023 Jun 26;25:e43349. doi: 10.2196/43349. J Med Internet Res. 2023. PMID: 37358900 Free PMC article.
-
Participatory Interventions for Sexual Health Promotion for Adolescents and Young Adults on the Internet: Systematic Review.J Med Internet Res. 2020 Jul 31;22(7):e15378. doi: 10.2196/15378. J Med Internet Res. 2020. PMID: 32735217 Free PMC article.
-
Mobile phone-based interventions for improving contraception use.Cochrane Database Syst Rev. 2023 Jul 17;7(7):CD011159. doi: 10.1002/14651858.CD011159.pub3. Cochrane Database Syst Rev. 2023. PMID: 37458240 Free PMC article.
-
Development of a Mobile Phone App to Promote Safe Sex Practice Among Youth in Stockholm, Sweden: Qualitative Study.JMIR Form Res. 2020 Jan 28;4(1):e12917. doi: 10.2196/12917. JMIR Form Res. 2020. PMID: 32012038 Free PMC article.
-
Randomized controlled trial protocol to evaluate the effect of an educational intervention using information, motivation and behavioral skills model on sexual satisfaction of new couples in Iran.Reprod Health. 2019 Nov 15;16(1):168. doi: 10.1186/s12978-019-0821-7. Reprod Health. 2019. PMID: 31730473 Free PMC article.
References
-
- World Health Organization: Global Health Sector Strategy on Sexually Transmitted Infections 2016–2021 In. Retrieved 10.10.16 from: http://apps.who.int/iris/bitstream/10665/246296/1/WHO-RHR-16.09-eng.pdf?...; 2016. - PubMed
-
- Nigro G, Mazzocco M, Mattia E, Di Renzo GC, Carta G, Anceschi MM. Role of the infections in recurrent spontaneous abortion. J Matern Fetal Neonatal Med. 2011;24(8):983–9. - PubMed
-
- ECDC: European Centre for Disease prevention and Control; Chlamydia Control in Eurpoe - a survey of Member States. In. Retrieved 21.03.16 from: http://ecdc.europa.eu/en/publications/Publications/chlamydia-control-sur...; 2012.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical