Effect of diagnosis related groups implementation on the intensive care unit of a Swiss tertiary hospital: a cohort study
- PMID: 29402271
- PMCID: PMC5800035
- DOI: 10.1186/s12913-018-2869-4
Effect of diagnosis related groups implementation on the intensive care unit of a Swiss tertiary hospital: a cohort study
Abstract
Background: In 2013 the Swiss Diagnosis Related Groups ((Swiss)-DRG) was implemented in Intensive Care Units (ICU). Its impact on hospitalizations has not yet been examined. We compared the number of ICU admissions, according to clinical severity and referring institution, and screened whether implementation of Swiss-DRG affected admission policy, ICU length-of-stay (ICU-LOS) or ICU mortality.
Methods: Retrospective, single centre, cohort study conducted at the University Hospital Zurich, Switzerland between January 2009 and end of September 2013. Demographic and clinical data was retrieved from a quality assurance database.
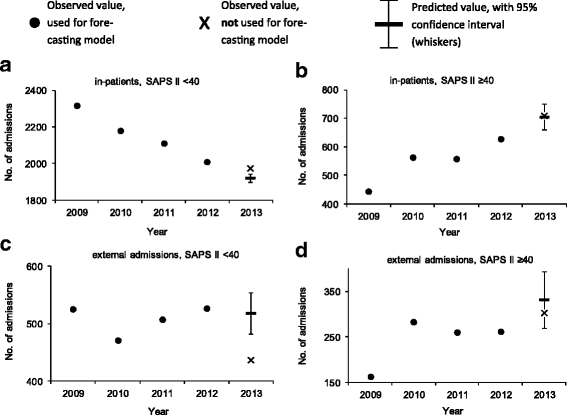
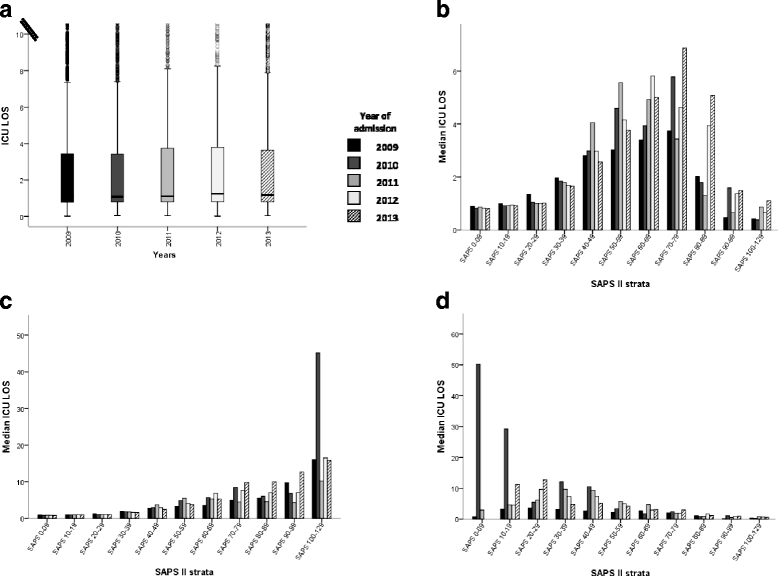
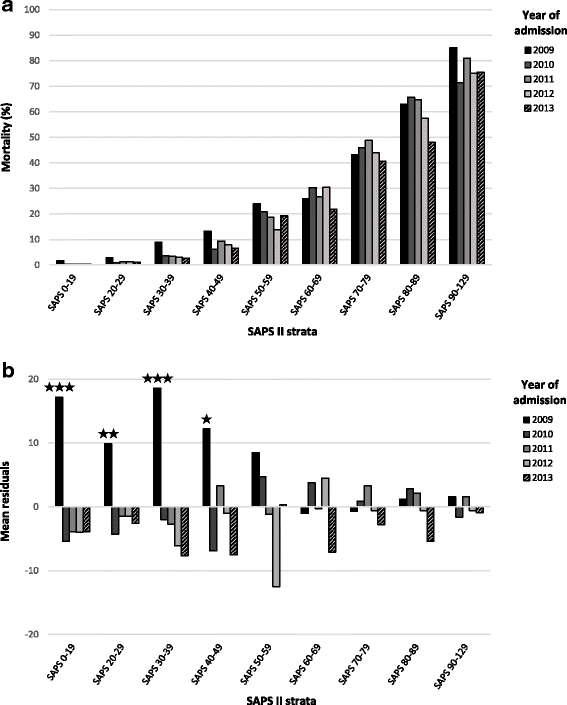
Results: Admissions (n = 17,231) before the introduction of Swiss-DRG were used to model expected admissions after DRG, and then compared to the observed admissions. Forecasting matched observations in patients with a high clinical severity admitted from internal units and external hospitals (admitted / predicted: 709 / 703, [95% Confidence Interval (CI), 658-748] and 302 / 332, [95% CI, 269-365] respectively). In patients with low severity of disease, in-house admissions became more frequent than expected and external admission were less frequent (admitted / predicted: 1972 / 1910, [95% CI, 1898-1940] and 436 / 518, [95% CI, 482-554] respectively). Various mechanisms related to Swiss-DRG may have led to these changes. DRG could not be linked to significant changes in regard to ICU-LOS and ICU mortality.
Conclusions: DRG introduction had not affected ICU admissions policy, except for an increase of in-house patients with a low clinical severity of disease. DRG had neither affected ICU mortality nor ICU-LOS.
Keywords: DRG; Diagnosis related groups; Epidemiology; ICU admissions; Switzerland.
Conflict of interest statement
Ethics approval and consent to participate
This observational trial complies with the current version of the Declaration of Helsinki and the national legal and regulatory requirements, and has been approved by the Canton Ethics Committee (Kantonale Ethikkommission Zurich, Switzerland, KEK-ZH-Nr. 2014–0452).
Consent for publication
According to the Ethics Committee no specific consent for the study was required given that the study was performed with an anonymized set of data i.e. without individual data.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Eidgenössisches Departement des Innern EDI - Bundesamt für Statistik BFS - Schweizerische Eidgenossenschaft. Gesundheitsstatistik 2012. Neuchâtel; 2012. https://www.bfs.admin.ch/bfsstatic/dam/assets/348071/master. Accessed 29 Jan 2018.
-
- Bundesamt für Statistik BFS - Website Statistik Schweiz. Kosten des Gesundheitswesens nach Leistungserbringern, Periode 1995–2012 (je-d-14.05.02.01). http://www.bfs.admin.ch/bfs/portal/de/index/themen/14/05/blank/key/leist.... Accessed 22 Jan 2015.
-
- Schweizerische Eidgenossenschaft . Bundesgesetz über die Krankenversicherung (KVG) (Spitalfinanzierung), Aenderung vom 21. Dezember 2007. 2007.
-
- Schweizerische Eidgenossenschaft - Bundesamt für Gesundheit BAG - Bern. Medienmitteilungen. https://www.admin.ch/gov/de/start/dokumentation/medienmitteilungen.msg-i.... Accessed 22 Jan 2015.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

