Establishment of a yeast-based VLP platform for antigen presentation
- PMID: 29402276
- PMCID: PMC5798182
- DOI: 10.1186/s12934-018-0868-0
Establishment of a yeast-based VLP platform for antigen presentation
Abstract
Background: Chimeric virus-like particles (VLP) allow the display of foreign antigens on their surface and have proved valuable in the development of safe subunit vaccines or drug delivery. However, finding an inexpensive production system and a VLP scaffold that allows stable incorporation of diverse, large foreign antigens are major challenges in this field.
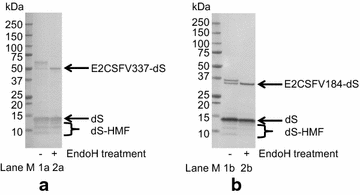
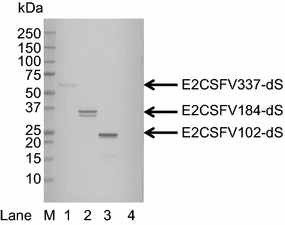
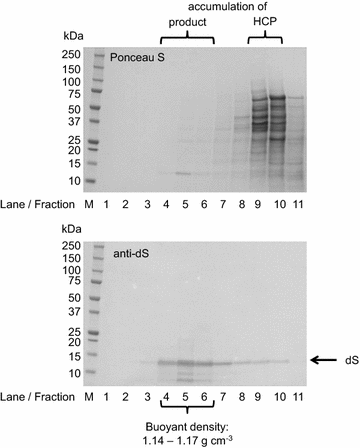
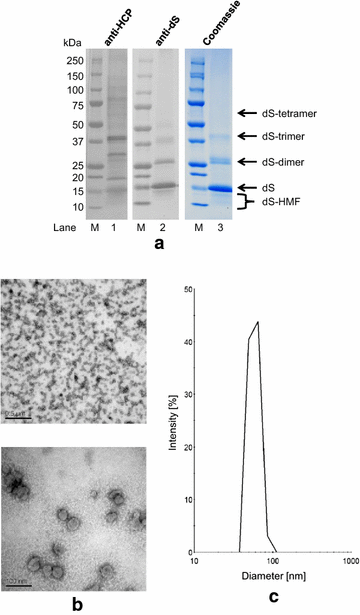
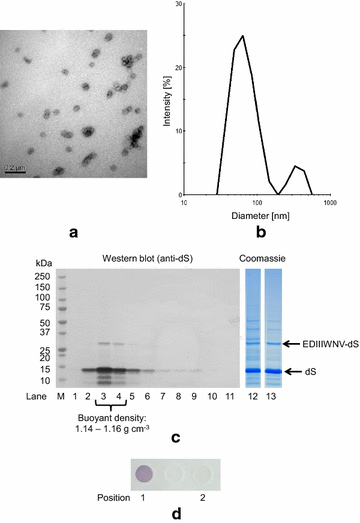
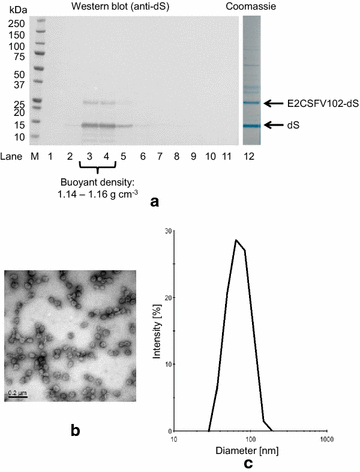
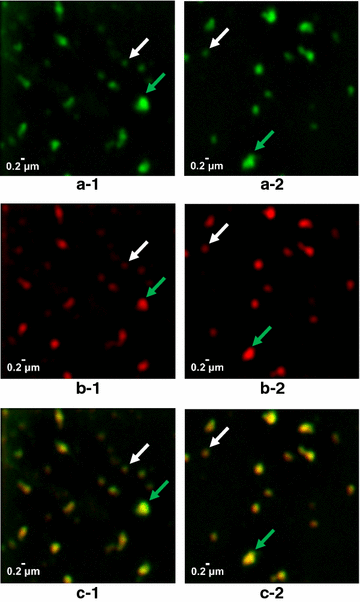
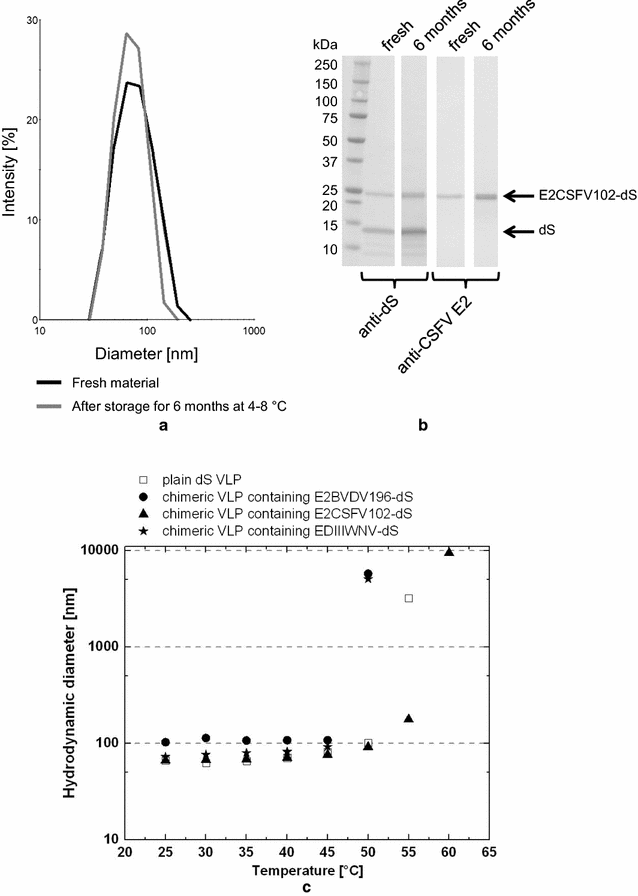
Results: In this study, a versatile and cost-effective platform for chimeric VLP development was established. The membrane integral small surface protein (dS) of the duck hepatitis B virus was chosen as VLP scaffold and the industrially applied and safe yeast Hansenula polymorpha (syn. Pichia angusta, Ogataea polymorpha) as the heterologous expression host. Eight different, large molecular weight antigens of up to 412 amino acids derived from four animal-infecting viruses were genetically fused to the dS and recombinant production strains were isolated. In all cases, the fusion protein was well expressed and upon co-production with dS, chimeric VLP containing both proteins could be generated. Purification was accomplished by a downstream process adapted from the production of a recombinant hepatitis B VLP vaccine. Chimeric VLP were up to 95% pure on protein level and contained up to 33% fusion protein. Immunological data supported surface exposure of the foreign antigens on the native VLP. Approximately 40 mg of chimeric VLP per 100 g dry cell weight could be isolated. This is highly comparable to values reported for the optimized production of human hepatitis B VLP. Purified chimeric VLP were shown to be essentially stable for 6 months at 4 °C.
Conclusions: The dS-based VLP scaffold tolerates the incorporation of a variety of large molecular weight foreign protein sequences. It is applicable for the display of highly immunogenic antigens originating from a variety of pathogens. The yeast-based production system allows cost-effective production that is not limited to small-scale fundamental research. Thus, the dS-based VLP platform is highly efficient for antigen presentation and should be considered in the development of future vaccines.
Keywords: Animal infectious diseases; Antibiotic-free; Antigen presentation; Chimeric virus-like particles; DHBV; Hansenula polymorpha; Pichia angusta; Virus-like particles.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

