Clinical results of proton beam therapy for elderly patients with non-small cell lung cancer
- PMID: 29402290
- PMCID: PMC5799978
- DOI: 10.1186/s13014-018-0967-4
Clinical results of proton beam therapy for elderly patients with non-small cell lung cancer
Abstract
Background: The purpose of the present study was to evaluate retrospectively the efficacy and safety of proton beam therapy for elderly patients (≥80 years of age) with non-small cell lung cancer.
Methods: Patients diagnosed with T1-4 N0 M0 non-small cell lung cancer and treated with proton beam therapy between January 2009 and 2015 were recruited from our database retrospectively. Toxicity was evaluated using The Common Terminology Criteria for Adverse Events version 4.0.
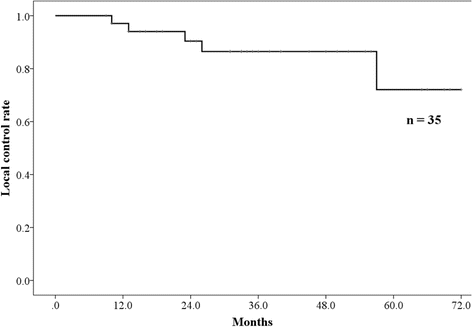
Results: Thirty-five patients, including 25 (71%) with clinically inoperable lung cancer, were administered proton beam therapy. The median age was 82 years (range: 80-87 years), and the median follow-up time was 34 months (range: 10-72 months). The median dose of proton beam therapy was 80.0 Gy relative biological effectiveness (RBE) (range: 60.0-80.0 Gy [RBE]), and all patients completed the treatments. All patients were followed for at least 23 months or until their death. The 3-year overall survival rate was 67.2% (90.0% in patients with operable lung cancer, and 58.2% in those with inoperable lung cancer). The 3-year local control rate was 86.5%. Two patients presented with grade 2 pneumonitis. The occurrence rate of grade 2 pneumonitis was significantly correlated with a high lung V20 (p = 0.030), and a high mean lung dose (p = 0.030), and a low ratio of lung volume spared from 0.05 Gy (RBE) dose (total lung volume minus lung volume irradiated at least 0.05 Gy [RBE]) (p = 0.030). However, there were no cases of grade 3 or higher radiation pneumonitis.
Conclusions: This study suggests that the proton beam therapy was feasible for elderly patients with non-small cell lung cancer and can be considered as one of the treatment choices for elderly patients with lung cancer.
Keywords: Elderly; Non-small cell lung cancer; Pneumonitis; Protons.
Conflict of interest statement
Ethics approval and consent to participate
We obtained general informed consent for the research from patients. Present retrospective study was approved by the ethics committees of our institution (approval number: D17–31). The study was conducted in accordance with the Declaration of Helsinki.
Consent for publication
We obtained written informed consent to publish from patients.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

