Sustainable reduction of antibiotic-induced antimicrobial resistance (ARena) in German ambulatory care: study protocol of a cluster randomised trial
- PMID: 29402306
- PMCID: PMC5800289
- DOI: 10.1186/s13012-018-0722-0
Sustainable reduction of antibiotic-induced antimicrobial resistance (ARena) in German ambulatory care: study protocol of a cluster randomised trial
Abstract
Background: Despite many initiatives to enhance the rational use of antibiotics, there remains substantial room for improvement. The overall aim of this study is to optimise the appropriate use of antibiotics in German ambulatory care in patients with acute non-complicated infections (respiratory tract infections, such as bronchitis, sinusitis, tonsillitis and otitis media), community-acquired pneumonia and non-complicated cystitis, in order to counter the advancing antimicrobial resistance development.
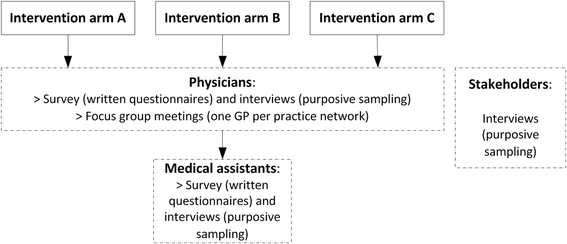
Methods: A three-armed cluster randomised trial will be conducted in 14 practice networks in two German federal states (Bavaria and North Rhine-Westphalia) and an added cohort that reflects standard care. The trial is accompanied by a process evaluation. Each arm will receive a different set of implementation strategies. Arm A receives a standard set, comprising of e-learning on communication with patients and quality circles with data-based feedback for physicians, information campaigns for the public, patient information material and performance-based additional reimbursement. Arm B receives this standard set plus e-learning on communication with patients and quality circles with data-based feedback tailored for non-physician health professionals of the practice team and information material for tablet computers (culture sensitive). Arm C receives the standard set as well as a computerised decision support system and quality circles in local multidisciplinary groups. The study aims to recruit 193 practices which will provide data on 23,934 patients each year (47,867 patients in total). The outcome evaluation is based on claims data and refers to established indicators of the European Surveillance of Antimicrobial Consumption Network (ESAC-Net). Primary and secondary outcomes relate to prescribing of antibiotics, which will be analysed in multivariate regression models. The process evaluation is based on interviews with surveys among physicians, non-physician health professionals of the practice team and stakeholders. A patient survey is conducted in one of the study arms. Interview data will be qualitatively analysed using thematic framework analysis. Survey data of physicians, non-physician health professionals of the practice team and patients will use descriptive and exploratory statistics for analysis.
Discussion: The ARena trial will examine the effectiveness of large scale implementation strategies and explore their delivery in routine practice.
Trial registration: ISRCTN, ISRCTN58150046 . Registered 24 August 2017.
Keywords: Ambulatory care; Antibiotics; Antimicrobial resistance; Germany; Implementation science; Inappropriate prescribing; Infection; Randomised trial.
Conflict of interest statement
Ethics approval and consent to participate
This study has received ethical approval by the ethics committee of the Medical Faculty of the University of Heidelberg (reference number S-353/2017) as well as the ethics committee of the Medical Association Baden-Wurttemberg (reference number B-F-2017-104).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interest. Joachim Szecsenyi holds stocks of the aQua-Institute.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Bundesministerium für Gesundheit. DART 2020. 2015. https://www.bundesgesundheitsministerium.de/fileadmin/Dateien/3_Download.... Accessed 15 May 2017.
-
- German College of General Practitioners and Family Physicians (DEGAM). Leitlinien der DEGAM. http://www.degam.de/degam-leitlinien-379.html. Accessed 11 Nov 2017.
-
- Little P, Stuart B, Francis N, Douglas E, Tonkin-Crine S, Anthierens S, et al. Effects of internet-based training on antibiotic prescribing rates for acute respiratory-tract infections: a multinational, cluster, randomised, factorial, controlled trial. Lancet. 2013;382:1175–1182. doi: 10.1016/S0140-6736(13)60994-0. - DOI - PMC - PubMed
-
- Anthierens S, Tonkin-Crine S, Cals JW, Coenen S, Yardley L, Brookes-Howell L, et al. Clinicians’ views and experiences of interventions to enhance the quality of antibiotic prescribing for acute respiratory tract infections. J Gen Intern Med. 2015;30:408–416. doi: 10.1007/s11606-014-3076-6. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical