The IMPROVE-GAP Trial aiming to improve evidence-based management of community-acquired pneumonia: study protocol for a stepped-wedge randomised controlled trial
- PMID: 29402313
- PMCID: PMC5800278
- DOI: 10.1186/s13063-017-2407-4
The IMPROVE-GAP Trial aiming to improve evidence-based management of community-acquired pneumonia: study protocol for a stepped-wedge randomised controlled trial
Abstract
Background: Community-acquired pneumonia is a leading worldwide cause of hospital admissions and healthcare resource consumption. The largest proportion of hospitalisations now occurs in older patients, with high rates of multimorbidity and complex care needs. In Australia, this population is usually managed by hospital inpatient general internal medicine units. Adherence to consensus best-practice guidelines is poor. Ensuring evidence-based care and reducing length of stay may improve patient outcomes and reduce organisational costs. This study aims to evaluate an alternative model of care designed to improve adherence to four Level 1 or 2 evidence-supported interventions (routine corticosteroids, early switch to oral antibiotics, early mobilisation and routine malnutrition screening).
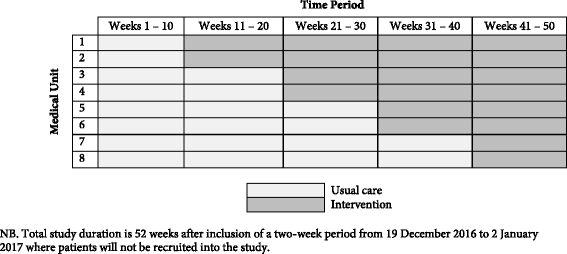
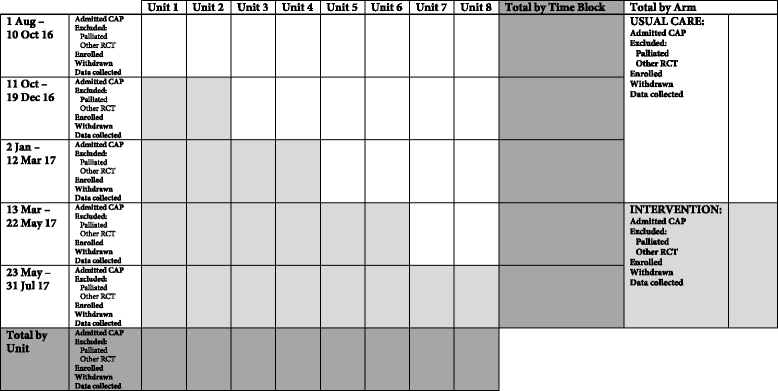
Methods/design: The IMPROVing Evidence-based treatment Gaps and outcomes in community-Acquired Pneumonia (IMPROVE-GAP) trial is a pragmatic, investigator-initiated, stepped-wedge randomised trial. Patients hospitalised under a general internal medicine unit who meet a standard case definition for community-acquired pneumonia will be included. Eight general internal medicine units at two Australian hospitals in a single health service will be randomised using concealed allocation to: (i) usual medical, nursing and allied health care delivered according to existing organisational practice or (ii) care supported by a dedicated "community-acquired pneumonia service": a multidisciplinary team deploying algorithm-based implementation of a bundle of the four evidence-based interventions. The primary outcome measure will be length of hospital stay. Secondary outcome measures include inpatient mortality, 30 and 90 day readmission rates and mortality and health-service utilisation costs. Protocol adherence will be measured and reported, and serious adverse events (rates of hyperglycaemia requiring new insulin; falls during mobilisation) will be collected and reported.
Discussion: IMPROVE-GAP represents an important and unique precedent for testing a new service-delivery model for improving compliance with a number of evidence-based interventions. Its stepped-wedge randomised controlled trial design provides a means to address some significant ethical, organisational and other methodological challenges to evaluating the effectiveness of health-service interventions in complex hospital populations. The new service-delivery model will effectively be fully implemented by trial completion, facilitating rapid, seamless translation into practice should care outcomes be superior. This trial is currently recruiting.
Trial registration: ClinicalTrials.gov, NCT02835040. Prospectively registered on 22 May 2016.
Keywords: Antibiotic; Community-acquired pneumonia; Corticosteroids; Early mobilisation; Malnutrition; Randomised controlled trial.
Conflict of interest statement
Ethics approval and consent to participate
The study protocol was approved by the hospital’s institutional review board (Melbourne Health Human Research Ethics Committee [Protocol reference: MH2016.014]). As part of the ethical approval, a waiver of the requirement for individual participant informed consent was sought and granted, as outlined in the study protocol.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


Similar articles
-
Can an antimicrobial stewardship program reduce length of stay of immune-competent adult patients admitted to hospital with diagnosis of community-acquired pneumonia? Study protocol for pragmatic controlled non-randomized clinical study.Trials. 2015 Aug 14;16:355. doi: 10.1186/s13063-015-0871-2. Trials. 2015. PMID: 26272324 Free PMC article. Clinical Trial.
-
The optimal antibiotic treatment duration for community-acquired pneumonia in adults diagnosed in general practice in Denmark (CAP-D): an open-label, pragmatic, randomised controlled trial.Trials. 2024 Sep 27;25(1):627. doi: 10.1186/s13063-024-08477-z. Trials. 2024. PMID: 39334468 Free PMC article.
-
Impact of disinvestment from weekend allied health services across acute medical and surgical wards: 2 stepped-wedge cluster randomised controlled trials.PLoS Med. 2017 Oct 31;14(10):e1002412. doi: 10.1371/journal.pmed.1002412. eCollection 2017 Oct. PLoS Med. 2017. PMID: 29088237 Free PMC article. Clinical Trial.
-
Evidence-based emergency medicine/critically appraised topic. Evidence behind the 4-hour rule for initiation of antibiotic therapy in community-acquired pneumonia.Ann Emerg Med. 2008 May;51(5):651-62, 662.e1-2. doi: 10.1016/j.annemergmed.2007.10.022. Epub 2008 Feb 13. Ann Emerg Med. 2008. PMID: 18272253 Review.
-
Adherence to guidelines for community-acquired pneumonia: does it decrease cost of care?Pharmacoeconomics. 2004;22(7):413-20. doi: 10.2165/00019053-200422070-00001. Pharmacoeconomics. 2004. PMID: 15137880 Review.
Cited by
-
Patient-reported outcome measurement in community-acquired pneumonia: feasibility of routine application in an elderly hospitalized population.Pilot Feasibility Stud. 2019 Jul 27;5:97. doi: 10.1186/s40814-019-0481-y. eCollection 2019. Pilot Feasibility Stud. 2019. PMID: 31372236 Free PMC article.
-
A novel counterbalanced implementation study design: methodological description and application to implementation research.Implement Sci. 2019 May 2;14(1):45. doi: 10.1186/s13012-019-0896-0. Implement Sci. 2019. PMID: 31046788 Free PMC article.
-
Feasibility and adherence to moderate intensity cardiovascular fitness training following stroke: a pilot randomized controlled trial.BMC Neurol. 2021 Mar 22;21(1):132. doi: 10.1186/s12883-021-02052-8. BMC Neurol. 2021. PMID: 33745454 Free PMC article. Clinical Trial.
References
-
- Global Burden of Disease Mortality Causes of Death Collaborators Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–71. doi: 10.1016/S0140-6736(14)61682-2. - DOI - PMC - PubMed
-
- Arnold FW, Wiemken TL, Peyrani P, Ramirez JA, Brock GN, Authors C. Mortality differences among hospitalized patients with community-acquired pneumonia in three world regions: results from the Community-Acquired Pneumonia Organization (CAPO) International Cohort Study. Respir Med. 2013;107:1101–11. doi: 10.1016/j.rmed.2013.04.003. - DOI - PubMed
-
- AIHW . Asthma, chronic obstructive pulmonary disease and other respiratory diseases in Australia. Canberra: AIHW; 2010.
-
- The Australian Lung Foundation . Respiratory infectious disease burden in Australia. Brisbane: The Australian Lung Foundation; 2007.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

