The CFTR-Associated Ligand Arrests the Trafficking of the Mutant ΔF508 CFTR Channel in the ER Contributing to Cystic Fibrosis
- PMID: 29402832
- PMCID: PMC5861351
- DOI: 10.1159/000487120
The CFTR-Associated Ligand Arrests the Trafficking of the Mutant ΔF508 CFTR Channel in the ER Contributing to Cystic Fibrosis
Abstract
Background/aims: The CFTR-Associated Ligand (CAL), a PDZ domain containing protein with two coiled-coil domains, reduces cell surface WT CFTR through degradation in the lysosome by a well-characterized mechanism. However, CAL's regulatory effect on ΔF508 CFTR has remained almost entirely uninvestigated.
Methods: In this study, we describe a previously unknown pathway for CAL by which it regulates the membrane expression of ΔF508 CFTR through arrest of ΔF508 CFTR trafficking in the endoplasmic reticulum (ER) using a combination of cell biology, biochemistry and electrophysiology.
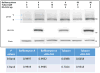
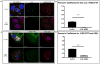
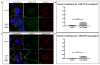
Results: We demonstrate that CAL is an ER localized protein that binds to ΔF508 CFTR and is degraded in the 26S proteasome. When CAL is inhibited, ΔF508 CFTR retention in the ER decreases and cell surface expression of mature functional ΔF508 CFTR is observed alongside of enhanced expression of plasma membrane scaffolding protein NHERF1. Chaperone proteins regulate this novel process, and ΔF508 CFTR binding to HSP40, HSP90, HSP70, VCP, and Aha1 changes to improve ΔF508 CFTR cell surface trafficking.
Conclusion: Our results reveal a pathway in which CAL regulates the cell surface availability and intracellular retention of ΔF508 CFTR.
Keywords: Endoplasmic reticulum; F508-del CFTR; Maturation; PDZ-domain; Trafficking.
© 2018 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
Figures







References
-
- Kerem BS, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, Buckwald M, Tsui LC. Identification of the cystic fibrosis gene: Genetic analysis. Sci. 1989;245:1073–1080. - PubMed
-
- Pilewski JM, Frizzell RA. Role of CFTR in airway disease. Physiol Rev. 1999;79:S215–S255. - PubMed
-
- Rosenstein BJ. Cystic fibrosis diagnosis: New dilemmas for an old disorder. Ped Pulm. 2002;33:83–84. - PubMed
-
- Sheppard DN, Welsh MJ. Structure and function of the CFTR chloride channel. Physiol Rev. 1999;79:S23–S45. - PubMed
-
- Sosnay PR, Siklosi KR, Van GF, Kaniecki K, Yu H, Sharma N, Ramalho AS, Amaral MD, Dorfman R, Zielenski J, Masica DL, Karchin R, Millen L, Thomas PJ, Patrinos GP, Corey M, Lewis MH, Rommens JM, Castellani C, Penland CM, Cutting GR. Defining the disease liability of variants in the cystic fibrosis transmembrane conductance regulator gene. Nat Genet. 2013;45:1160–1167. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
