Steric exclusion and protein conformation determine the localization of plasma membrane transporters
- PMID: 29402931
- PMCID: PMC5799302
- DOI: 10.1038/s41467-018-02864-2
Steric exclusion and protein conformation determine the localization of plasma membrane transporters
Abstract
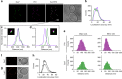
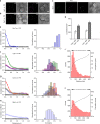
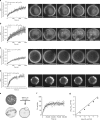
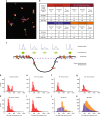
The plasma membrane (PM) of Saccharomyces cerevisiae contains membrane compartments, MCC/eisosomes and MCPs, named after the protein residents Can1 and Pma1, respectively. Using high-resolution fluorescence microscopy techniques we show that Can1 and the homologous transporter Lyp1 are able to diffuse into the MCC/eisosomes, where a limited number of proteins are conditionally trapped at the (outer) edge of the compartment. Upon addition of substrate, the immobilized proteins diffuse away from the MCC/eisosomes, presumably after taking a different conformation in the substrate-bound state. Our data indicate that the mobile fraction of all integral plasma membrane proteins tested shows extremely slow Brownian diffusion through most of the PM. We also show that proteins with large cytoplasmic domains, such as Pma1 and synthetic chimera of Can1 and Lyp1, are excluded from the MCC/eisosomes. We hypothesize that the distinct localization patterns found for these integral membrane proteins in S. cerevisiae arises from a combination of slow lateral diffusion, steric exclusion, and conditional trapping in membrane compartments.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

