The selective reversible FAAH inhibitor, SSR411298, restores the development of maladaptive behaviors to acute and chronic stress in rodents
- PMID: 29403000
- PMCID: PMC5799259
- DOI: 10.1038/s41598-018-20895-z
The selective reversible FAAH inhibitor, SSR411298, restores the development of maladaptive behaviors to acute and chronic stress in rodents
Abstract
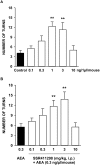
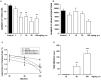
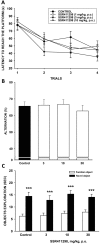
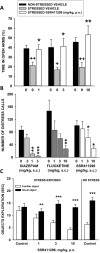
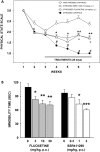
Enhancing endogenous cannabinoid (eCB) signaling has been considered as a potential strategy for the treatment of stress-related conditions. Fatty acid amide hydrolase (FAAH) represents the primary degradation enzyme of the eCB anandamide (AEA), oleoylethanolamide (OEA) and palmitoylethanolamide (PEA). This study describes a potent reversible FAAH inhibitor, SSR411298. The drug acts as a selective inhibitor of FAAH, which potently increases hippocampal levels of AEA, OEA and PEA in mice. Despite elevating eCB levels, SSR411298 did not mimic the interoceptive state or produce the behavioral side-effects (memory deficit and motor impairment) evoked by direct-acting cannabinoids. When SSR411298 was tested in models of anxiety, it only exerted clear anxiolytic-like effects under highly aversive conditions following exposure to a traumatic event, such as in the mouse defense test battery and social defeat procedure. Results from experiments in models of depression showed that SSR411298 produced robust antidepressant-like activity in the rat forced-swimming test and in the mouse chronic mild stress model, restoring notably the development of inadequate coping responses to chronic stress. This preclinical profile positions SSR411298 as a promising drug candidate to treat diseases such as post-traumatic stress disorder, which involves the development of maladaptive behaviors.
Conflict of interest statement
Organizations from whom the authors have received compensation for professional services: Guy Griebel, employee of Sanofi; Philippe Pichat, employee of Sanofi; Jeanne Stemmelin, employee of Sanofi; Mati Lopez-Grancha, employee of Sanofi; Valérie Fauchey, employee of Sanofi; Franck Slowinski, employee of Sanofi; Gihad Dargazanli, employee of Sanofi, Ahmed Abouabdellah, employee of Sanofi; Caroline Cohen, employee of Sanofi; Olivier E. Bergis, employee of Sanofi.
Figures






Similar articles
-
Antidepressants and changes in concentration of endocannabinoids and N-acylethanolamines in rat brain structures.Neurotox Res. 2014 Aug;26(2):190-206. doi: 10.1007/s12640-014-9465-0. Epub 2014 Mar 21. Neurotox Res. 2014. PMID: 24652522 Free PMC article.
-
Full inhibition of spinal FAAH leads to TRPV1-mediated analgesic effects in neuropathic rats and possible lipoxygenase-mediated remodeling of anandamide metabolism.PLoS One. 2013;8(4):e60040. doi: 10.1371/journal.pone.0060040. Epub 2013 Apr 3. PLoS One. 2013. PMID: 23573230 Free PMC article.
-
Lack of effect of chronic pre-treatment with the FAAH inhibitor URB597 on inflammatory pain behaviour: evidence for plastic changes in the endocannabinoid system.Br J Pharmacol. 2012 Oct;167(3):627-40. doi: 10.1111/j.1476-5381.2012.02028.x. Br J Pharmacol. 2012. PMID: 22595021 Free PMC article.
-
Amygdala FAAH and anandamide: mediating protection and recovery from stress.Trends Pharmacol Sci. 2013 Nov;34(11):637-44. doi: 10.1016/j.tips.2013.08.008. Epub 2013 Oct 25. Trends Pharmacol Sci. 2013. PMID: 24325918 Free PMC article. Review.
-
Fatty acid amide hydrolase: biochemistry, pharmacology, and therapeutic possibilities for an enzyme hydrolyzing anandamide, 2-arachidonoylglycerol, palmitoylethanolamide, and oleamide.Biochem Pharmacol. 2001 Sep 1;62(5):517-26. doi: 10.1016/s0006-2952(01)00712-2. Biochem Pharmacol. 2001. PMID: 11585048 Review.
Cited by
-
Structure-activity relationship studies of benzothiazole-phenyl analogs as multi-target ligands to alleviate pain without affecting normal behavior.Prostaglandins Other Lipid Mediat. 2023 Feb;164:106702. doi: 10.1016/j.prostaglandins.2022.106702. Epub 2022 Dec 16. Prostaglandins Other Lipid Mediat. 2023. PMID: 36529320 Free PMC article.
-
NAPE-PLD deletion in stress-TRAPed neurons results in an anxiogenic phenotype.Transl Psychiatry. 2023 May 6;13(1):152. doi: 10.1038/s41398-023-02448-9. Transl Psychiatry. 2023. PMID: 37149657 Free PMC article.
-
FAAH Modulators from Natural Sources: A Collection of New Potential Drugs.Cells. 2025 Apr 5;14(7):551. doi: 10.3390/cells14070551. Cells. 2025. PMID: 40214504 Free PMC article. Review.
-
Altered endocannabinoid metabolism compromises the brain-CSF barrier and exacerbates chronic deficits after traumatic brain injury in mice.Exp Neurol. 2023 Mar;361:114320. doi: 10.1016/j.expneurol.2023.114320. Epub 2023 Jan 7. Exp Neurol. 2023. PMID: 36627040 Free PMC article.
-
The endocannabinoid system as a putative target for the development of novel drugs for the treatment of psychiatric illnesses.Psychol Med. 2023 Nov;53(15):7006-7024. doi: 10.1017/S0033291723002465. Epub 2023 Sep 6. Psychol Med. 2023. PMID: 37671673 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

