Bispecific antibodies: design, therapy, perspectives
- PMID: 29403265
- PMCID: PMC5784585
- DOI: 10.2147/DDDT.S151282
Bispecific antibodies: design, therapy, perspectives
Abstract
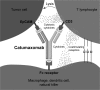
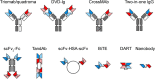
Antibodies (Abs) containing two different antigen-binding sites in one molecule are called bispecific. Bispecific Abs (BsAbs) were first described in the 1960s, the first monoclonal BsAbs were generated in the 1980s by hybridoma technology, and the first article describing the therapeutic use of BsAbs was published in 1992, but the number of papers devoted to BsAbs has increased significantly in the last 10 years. Particular interest in BsAbs is due to their therapeutic use. In the last decade, two BsAbs - catumaxomab in 2009 and blinatumomab in 2014, were approved for therapeutic use. Papers published in recent years have been devoted to various methods of BsAb generation by genetic engineering and chemical conjugation, and describe preclinical and clinical trials of these drugs in a variety of diseases. This review considers diverse BsAb-production methods, describes features of therapeutic BsAbs approved for medical use, and summarizes the prospects of practical application of promising new BsAbs.
Keywords: bispecific antibodies; monoclonal antibodies; therapeutic antibodies.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures

References
-
- Spiess C, Zhai Q, Carter PJ. Alternative molecular formats and therapeutic applications for bispecific antibodies. Mol Immunol. 2015;67(2 Pt A):95–106. - PubMed
-
- Shima M, Hanabusa H, Taki M, et al. Factor VIII-mimetic function of humanized bispecific antibody in hemophilia A. N Engl J Med. 2016;374(21):2044–2053. - PubMed
-
- Yu YJ, Atwal JK, Zhang Y, et al. Therapeutic bispecific antibodies cross the blood-brain barrier in nonhuman primates. Sci Transl Med. 2014;6(261):261ra154. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

