Development of biocompatible and VEGF-targeted paclitaxel nanodrugs on albumin and graphene oxide dual-carrier for photothermal-triggered drug delivery in vitro and in vivo
- PMID: 29403275
- PMCID: PMC5777379
- DOI: 10.2147/IJN.S150977
Development of biocompatible and VEGF-targeted paclitaxel nanodrugs on albumin and graphene oxide dual-carrier for photothermal-triggered drug delivery in vitro and in vivo
Abstract
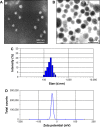
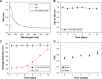
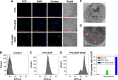
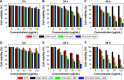
In this study, we performed the characterization and synthesis of biocompatible and targeted albumin and graphene oxide (GO) dual-carrier paclitaxel (PTX) nanoparticles for photothermal-triggered tumor therapy. PTX absorbed on GO nanosheets as cores were coated with human serum albumin (HSA), following surface conjugation with monoclonal antibodies (mAb) against vascular endothelial growth factor (VEGF; denoted as mAbVEGF) via polyethylene glycol linker to form targeted nanoparticles (PTX-GHP-VEGF). The spherical nanoparticles were 191±5 nm in size with good stability and biocompatibility. GO functioned as the first carrier and a near infrared absorber that can generate photothermal effects under 5-minute 808-nm laser irradiation to thermal trigger the release of PTX from the second carrier HSA nanoparticles. The mechanism of thermal-triggered drug release was also investigated preliminarily, in which the heat generated by GO induced swelling of PTX-GHP-VEGF nanoparticles which released the drugs. In vitro studies found that PTX-GHP-VEGF can efficiently target human SW-13 adrenocortical carcinoma cells as evaluated by confocal fluorescence microscopy as well as transmission electron microscopy, and showed an obvious thermal-triggered antitumor effect, mediated by apoptosis. Moreover, PTX-GHP-VEGF combined with near infrared irradiation showed specific tumor suppression effects with high survival rate after 100 days of treatment. PTX-GHP-VEGF also demonstrated high biosafety with no adverse effects on normal tissues and organs. These results highlight the remarkable potential of PTX-GHP-VEGF in photothermal controllable tumor treatment.
Keywords: graphene oxide; human serum albumin; paclitaxel; photothermal-triggered tumor therapy; tumor targeting.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures








References
-
- Xu Y, Zhu Y, Shen Z, et al. Significance of heparanase-1 and vascular endothelial growth factor in adrenocortical carcinoma angiogenesis: potential for therapy. Endocrine. 2011;40(3):445–451. - PubMed
-
- Terzolo M, Zaggia B, Allasino B, De Francia S. Practical treatment using mitotane for adrenocortical carcinoma. Curr Opin Endocrinol Diabetes Obes. 2014;21(3):159–165. - PubMed
-
- Germano A, Rapa I, Volante M, et al. Cytotoxic activity of gemcitabine, alone or in combination with mitotane, in adrenocortical carcinoma cell lines. Mol Cell Endocrinol. 2014;382(1):1–7. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

