Hypothalamic Alterations in Neurodegenerative Diseases and Their Relation to Abnormal Energy Metabolism
- PMID: 29403354
- PMCID: PMC5780436
- DOI: 10.3389/fnmol.2018.00002
Hypothalamic Alterations in Neurodegenerative Diseases and Their Relation to Abnormal Energy Metabolism
Abstract
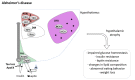
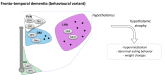
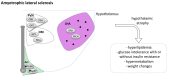
Neurodegenerative diseases (NDDs) are disorders characterized by progressive deterioration of brain structure and function. Selective neuronal populations are affected leading to symptoms which are prominently motor in amyotrophic lateral sclerosis (ALS) or Huntington's disease (HD), or cognitive in Alzheimer's disease (AD) and fronto-temporal dementia (FTD). Besides the common existence of neuronal loss, NDDs are also associated with metabolic changes such as weight gain, weight loss, loss of fat mass, as well as with altered feeding behavior. Importantly, preclinical research as well as clinical studies have demonstrated that altered energy homeostasis influences disease progression in ALS, AD and HD, suggesting that identification of the pathways leading to perturbed energy balance might provide valuable therapeutic targets Signals from both the periphery and central inputs are integrated in the hypothalamus, a major hub for the control of energy balance. Recent research identified major hypothalamic changes in multiple NDDs. Here, we review these hypothalamic alterations and seek to identify commonalities and differences in hypothalamic involvement between the different NDDs. These hypothalamic defects could be key in the development of perturbations in energy homeostasis in NDDs and further understanding of the underlying mechanisms might open up new avenues to not only treat weight loss but also to ameliorate overall neurological symptoms.
Keywords: Alzheimer’s disease; Huntington’s disease; amyotrophic lateral sclerosis; fronto-temporal dementia; hypothalamus; neurodegeneration; weight loss.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

