Contribution of Bicarbonate Assimilation to Carbon Pool Dynamics in the Deep Mediterranean Sea and Cultivation of Actively Nitrifying and CO2-Fixing Bathypelagic Prokaryotic Consortia
- PMID: 29403458
- PMCID: PMC5780414
- DOI: 10.3389/fmicb.2018.00003
Contribution of Bicarbonate Assimilation to Carbon Pool Dynamics in the Deep Mediterranean Sea and Cultivation of Actively Nitrifying and CO2-Fixing Bathypelagic Prokaryotic Consortia
Abstract
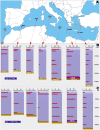
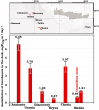
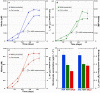
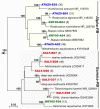
Covering two-thirds of our planet, the global deep ocean plays a central role in supporting life on Earth. Among other processes, this biggest ecosystem buffers the rise of atmospheric CO2. Despite carbon sequestration in the deep ocean has been known for a long time, microbial activity in the meso- and bathypelagic realm via the "assimilation of bicarbonate in the dark" (ABD) has only recently been described in more details. Based on recent findings, this process seems primarily the result of chemosynthetic and anaplerotic reactions driven by different groups of deep-sea prokaryoplankton. We quantified bicarbonate assimilation in relation to total prokaryotic abundance, prokaryotic heterotrophic production and respiration in the meso- and bathypelagic Mediterranean Sea. The measured ABD values, ranging from 133 to 370 μg C m-3 d-1, were among the highest ones reported worldwide for similar depths, likely due to the elevated temperature of the deep Mediterranean Sea (13-14°C also at abyssal depths). Integrated over the dark water column (≥200 m depth), bicarbonate assimilation in the deep-sea ranged from 396 to 873 mg C m-2 d-1. This quantity of produced de novo organic carbon amounts to about 85-424% of the phytoplankton primary production and covers up to 62% of deep-sea prokaryotic total carbon demand. Hence, the ABD process in the meso- and bathypelagic Mediterranean Sea might substantially contribute to the inorganic and organic pool and significantly sustain the deep-sea microbial food web. To elucidate the ABD key-players, we established three actively nitrifying and CO2-fixing prokaryotic enrichments. Consortia were characterized by the co-occurrence of chemolithoautotrophic Thaumarchaeota and chemoheterotrophic proteobacteria. One of the enrichments, originated from Ionian bathypelagic waters (3,000 m depth) and supplemented with low concentrations of ammonia, was dominated by the Thaumarchaeota "low-ammonia-concentration" deep-sea ecotype, an enigmatic and ecologically important group of organisms, uncultured until this study.
Keywords: ammonium-oxidizing Thaumarchaeota; anaplerotic reactions; dark bicarbonate assimilation; deep-sea microbial community; mediterranean sea.
Figures


Similar articles
-
Heterotrophic bicarbonate assimilation is the main process of de novo organic carbon synthesis in hadal zone of the Hellenic Trench, the deepest part of Mediterranean Sea.Environ Microbiol Rep. 2014 Dec;6(6):709-22. doi: 10.1111/1758-2229.12192. Environ Microbiol Rep. 2014. PMID: 25756124
-
Uptake-release dynamics of the inorganic and organic carbon pool mediated by planktonic prokaryotes in the deep Mediterranean Sea.Environ Microbiol. 2017 Mar;19(3):1163-1175. doi: 10.1111/1462-2920.13641. Epub 2017 Feb 6. Environ Microbiol. 2017. PMID: 28026100
-
Shifts in the meso- and bathypelagic archaea communities composition during recovery and short-term handling of decompressed deep-sea samples.Environ Microbiol Rep. 2015 Jun;7(3):450-9. doi: 10.1111/1758-2229.12272. Epub 2015 Apr 8. Environ Microbiol Rep. 2015. PMID: 25682761
-
Protist diversity and function in the dark ocean - Challenging the paradigms of deep-sea ecology with special emphasis on foraminiferans and naked protists.Eur J Protistol. 2020 Aug;75:125721. doi: 10.1016/j.ejop.2020.125721. Epub 2020 Jun 4. Eur J Protistol. 2020. PMID: 32575029 Review.
-
The Minderoo-Monaco Commission on Plastics and Human Health.Ann Glob Health. 2023 Mar 21;89(1):23. doi: 10.5334/aogh.4056. eCollection 2023. Ann Glob Health. 2023. PMID: 36969097 Free PMC article. Review.
Cited by
-
Comparative study of lysine acetylation in Vesicomyidae clam Archivesica marissinica and the manila clam Ruditapes philippinarum: adaptation mechanisms in cold seep environments.BMC Genomics. 2024 Oct 28;25(1):1006. doi: 10.1186/s12864-024-10916-9. BMC Genomics. 2024. PMID: 39465380 Free PMC article.
-
Grand Challenges in Microbe-Driven Marine Carbon Cycling Research.Front Microbiol. 2020 Jun 23;11:1039. doi: 10.3389/fmicb.2020.01039. eCollection 2020. Front Microbiol. 2020. PMID: 32655507 Free PMC article. No abstract available.
-
Carbon Fixation by Marine Ultrasmall Prokaryotes.Genome Biol Evol. 2019 Apr 1;11(4):1166-1177. doi: 10.1093/gbe/evz050. Genome Biol Evol. 2019. PMID: 30903144 Free PMC article.
-
Deep ocean metagenomes provide insight into the metabolic architecture of bathypelagic microbial communities.Commun Biol. 2021 May 21;4(1):604. doi: 10.1038/s42003-021-02112-2. Commun Biol. 2021. PMID: 34021239 Free PMC article.
References
-
- Arístegui J., Duarte C. M., Gasol J. M., Alonso-Sáez L. (2005). Active mesopelagic prokaryotes support high respiration in the subtropical northeast Atlantic Ocean. Geophys. Res. Lett. 32:L03608 10.1029/2004GL021863 - DOI
-
- Arístegui J., Gasol J. M., Duarte C. M., Herndld G. J. (2009). Microbial oceanography of the dark ocean's pelagic realm. Limnol. Oceanogr. 54, 1501–1529. 10.4319/lo.2009.54.5.1501 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

