TRAIL-Mediated Suppression of T Cell Receptor Signaling Inhibits T Cell Activation and Inflammation in Experimental Autoimmune Encephalomyelitis
- PMID: 29403497
- PMCID: PMC5786528
- DOI: 10.3389/fimmu.2018.00015
TRAIL-Mediated Suppression of T Cell Receptor Signaling Inhibits T Cell Activation and Inflammation in Experimental Autoimmune Encephalomyelitis
Abstract
Objective: Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) induces cell apoptosis by transducing apoptosis signals after interacting with its receptor (TRAIL-R). Although the actual biological role of TRAIL remains to be elucidated, recent accumulating evidence implies that TRAIL regulates immune responses and immune cell homeostasis via an apoptosis-independent pathway, suggesting a novel immune-regulatory role of TRAIL in autoimmune diseases. The purpose of this study is to address the immune-regulatory role and molecular mechanism of TRAIL in regulating T cell activation in autoimmune diseases.
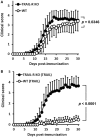
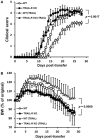
Design: TRAIL was administered to mice to induce experimental autoimmune encephalomyelitis (EAE), and to evaluate its impact on neuroinflammation and disease activity. The effects of TRAIL on neuroantigen [myelin oligodendrocyte glycoprotein (MOG)35-55]-activated T cell proliferation and cytokine production were investigated. TRAIL-treated MOG35-55-activated splenic Th17 cells were further adoptively transferred into Rag1 KO mice to induce passive EAE. Gene expression profiles of CD4+ T cells from EAE mice treated with TRAIL were analyzed by RNA sequencing and transcriptome analysis.
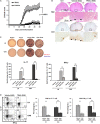
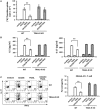
Results: TRAIL suppressed autoimmune encephalomyelitis and inhibited T cell reactivity to neuro-antigen in murine EAE, and the effects were dependent on TRAIL-R signaling. Moreover, TRAIL directly inhibited activation of MOG35-55-activated CD4+ T cells, resulting in suppression of neuroinflammation and reduced disease activity in adoptive transfer-induced EAE. Furthermore, TRAIL-R signaling inhibited phosphorylation of proximal T cell receptor (TCR)-associated tyrosine kinases in activated CD4+ T cells. Importantly, TRAIL/TRAIL-R interaction downregulated TCR downstream signaling genes in RNA sequencing and transcriptome analysis.
Conclusion: TRAIL/TRAIL-R interaction regulates CD4+ T cell activation in autoimmune inflammation and directly suppresses T cell activation via inhibiting TCR signaling, suggesting that TRAIL-R serves as a novel immune checkpoint in T cell responses.
Keywords: T cell activation; T cell receptor signaling; TRAIL; apoptosis; experimental autoimmune encephalomyelitis.
Figures

Similar articles
-
Prevention of experimental autoimmune encephalomyelitis by transfer of embryonic stem cell-derived dendritic cells expressing myelin oligodendrocyte glycoprotein peptide along with TRAIL or programmed death-1 ligand.J Immunol. 2005 Feb 15;174(4):1888-97. doi: 10.4049/jimmunol.174.4.1888. J Immunol. 2005. PMID: 15699115
-
Involvement of regulatory T cells in the experimental autoimmune encephalomyelitis-preventive effect of dendritic cells expressing myelin oligodendrocyte glycoprotein plus TRAIL.J Immunol. 2007 Jan 15;178(2):918-25. doi: 10.4049/jimmunol.178.2.918. J Immunol. 2007. PMID: 17202353
-
Roles of TNF-related apoptosis-inducing ligand in experimental autoimmune encephalomyelitis.J Immunol. 2001 Jan 15;166(2):1314-9. doi: 10.4049/jimmunol.166.2.1314. J Immunol. 2001. PMID: 11145715
-
Regulation of Interleukin-6 Receptor Signaling by TNF Receptor-Associated Factor 2 and 5 During Differentiation of Inflammatory CD4+ T Cells.Front Immunol. 2018 Aug 30;9:1986. doi: 10.3389/fimmu.2018.01986. eCollection 2018. Front Immunol. 2018. PMID: 30214449 Free PMC article. Review.
-
Regulatory T cells in spontaneous autoimmune encephalomyelitis.Immunol Rev. 2001 Aug;182:122-34. doi: 10.1034/j.1600-065x.2001.1820110.x. Immunol Rev. 2001. PMID: 11722629 Review.
Cited by
-
Apoptotic cell death in disease-Current understanding of the NCCD 2023.Cell Death Differ. 2023 May;30(5):1097-1154. doi: 10.1038/s41418-023-01153-w. Epub 2023 Apr 26. Cell Death Differ. 2023. PMID: 37100955 Free PMC article. Review.
-
The Role of TRAIL/DRs in the Modulation of Immune Cells and Responses.Cancers (Basel). 2019 Sep 30;11(10):1469. doi: 10.3390/cancers11101469. Cancers (Basel). 2019. PMID: 31574961 Free PMC article. Review.
-
A novel model based on necroptosis to assess progression for polycystic ovary syndrome and identification of potential therapeutic drugs.Front Endocrinol (Lausanne). 2023 Sep 7;14:1193992. doi: 10.3389/fendo.2023.1193992. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37745699 Free PMC article.
-
A transcriptomic study of probenecid on injured spinal cords in mice.PeerJ. 2020 Jan 3;8:e8367. doi: 10.7717/peerj.8367. eCollection 2020. PeerJ. 2020. PMID: 31921518 Free PMC article.
-
TRAIL and FasL Functions in Cancer and Autoimmune Diseases: Towards an Increasing Complexity.Cancers (Basel). 2019 May 8;11(5):639. doi: 10.3390/cancers11050639. Cancers (Basel). 2019. PMID: 31072029 Free PMC article. Review.
References
-
- Wu GS, Burns TF, Zhan Y, Alnemri ES, El-Deiry WS. Molecular cloning and functional analysis of the mouse homologue of the KILLER/DR5 tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) death receptor. Cancer Res (1999) 59(12):2770–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

