Two Distinct Pathways in Mice Generate Antinuclear Antigen-Reactive B Cell Repertoires
- PMID: 29403498
- PMCID: PMC5786517
- DOI: 10.3389/fimmu.2018.00016
Two Distinct Pathways in Mice Generate Antinuclear Antigen-Reactive B Cell Repertoires
Abstract
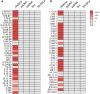
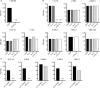
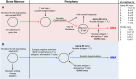
The escape of anti-self B cells from tolerance mechanisms like clonal deletion, receptor editing, and anergy results in the production of autoantibodies, which is a hallmark of many autoimmune disorders. In this study, we demonstrate that both germline sequences and somatic mutations contribute to autospecificity of B cell clones. For this issue, we investigated the development of antinuclear autoantibodies (ANAs) and their repertoire in two different mouse models. First, in aging mice that were shown to gain several autoimmune features over time including ANAs. Second, in mice undergoing a chronic graft-versus-host disease (GVHD), thereby developing systemic lupus erythematosus-like symptoms. Detailed repertoire analysis revealed that somatic hypermutations (SHM) were present in all Vh and practically all Vl regions of ANAs generated in these two models. The ANA B cell repertoire in aging mice was restricted, dominated by clonally related Vh1-26/Vk4-74 antibodies. In the collection of GVHD-derived ANAs, the repertoire was less restricted, but the usage of the Vh1-26/Vk4-74 combination was still apparent. Germline conversion showed that the SHM in the 4-74 light chain are deterministic for autoreactivity. Detailed analysis revealed that antinuclear reactivity of these antibodies could be induced by a single amino acid substitution in the CDR1 of the Vk4-74. In both aging B6 and young GVHD mice, conversion of the somatic mutations in the Vh and Vl regions of non Vh1-26/Vk4-74 using antibodies showed that B cells with a germline-encoded V gene could also contribute to the ANA-reactive B cell repertoire. These findings indicate that two distinct pathways generate ANA-producing B cells in both model systems. In one pathway, they are generated by Vh1-26/Vk4-74 expressing B cells in the course of immune responses to an antigen that is neither a nuclear antigen nor any other self-antigen. In the other pathway, ANA-producing B cells are derived from progenitors in the bone marrow that express B cell receptors (BCRs), which bind to nuclear antigens and that escape tolerance induction, possibly as a result of crosslinking of their BCRs by multivalent determinants of nuclear antigens.
Keywords: antinuclear antibodies; autoantibodies; monoclonal antibodies; mouse model; somatic hypermutation; systemic lupus erythematosus-like disease.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

