Quantification of desloratadine in human plasma by LC-ESI-MS/MS and application to a pharmacokinetic study
- PMID: 29403740
- PMCID: PMC5760887
- DOI: 10.1016/j.jpha.2012.01.008
Quantification of desloratadine in human plasma by LC-ESI-MS/MS and application to a pharmacokinetic study
Abstract
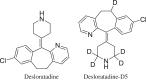
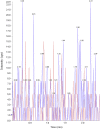
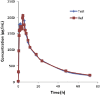
A simple, sensitive, and specific liquid chromatography tandem mass spectrometry (LC-MS/MS) method was developed for the quantification of desloratadine (DL) in human plasma using desloratadine-d5 (DLD5) as an internal standard (IS). Chromatographic separation was performed using an Xbridge C18 column (50 mm×4.6 mm, 5 μm) with an isocratic mobile phase composed of 10 mM ammonium formate: methanol (20:80, v/v), at a flow rate of 0.7 mL/min. DL and DLD5 were detected with proton adducts at m/z 311.2→259.2 and 316.2→264.3 in multiple reaction monitoring (MRM) positive modes, respectively. Liquid-liquid extraction (LLE) method was used to extract the drug and the IS. The method was validated over a linear concentration range of 5.0-5000.0 pg/mL with a correlation coefficient of (r2)≥0.9994. This method demonstrated intra- and inter-day precision within 0.7-2.0% and 0.7-2.7%, and an accuracy within 101.4-102.4%, and 99.5-104.8%. DL was found to be stable throughout the freeze-thaw cycles, bench-top, and postoperative stability studies. This method was successfully applied in the analysis of plasma samples following oral administration of DL (5 mg) in 35 healthy Indian male human volunteers under fasting conditions.
Keywords: Bioequivalence; Desloratadine; Mass spectrometry; Pharmacokinetic study.
Figures



Similar articles
-
Bioanalytical method development and validation of milnacipran in rat plasma by LC-MS/MS detection and its application to a pharmacokinetic study.J Pharm Anal. 2013 Dec;3(6):481-488. doi: 10.1016/j.jpha.2013.03.009. Epub 2013 Apr 29. J Pharm Anal. 2013. PMID: 29403859 Free PMC article.
-
A validated LC-MS/MS method for the determination of tolterodine and its metabolite in rat plasma and application to pharmacokinetic study.J Pharm Anal. 2013 Dec;3(6):489-499. doi: 10.1016/j.jpha.2013.04.005. Epub 2013 May 24. J Pharm Anal. 2013. PMID: 29403860 Free PMC article.
-
LC-ESI-MS/MS method for the quantification of entecavir in human plasma and its application to bioequivalence study.J Chromatogr B Analyt Technol Biomed Life Sci. 2011 Apr 1;879(11-12):769-76. doi: 10.1016/j.jchromb.2011.02.023. Epub 2011 Feb 21. J Chromatogr B Analyt Technol Biomed Life Sci. 2011. PMID: 21397572
-
Novel LC- ESI-MS/MS method for desvenlafaxine estimation human plasma: application to pharmacokinetic study.Biomed Chromatogr. 2016 Feb;30(2):249-55. doi: 10.1002/bmc.3542. Epub 2015 Aug 17. Biomed Chromatogr. 2016. PMID: 26095112
-
Method development and validation of montelukast in human plasma by HPLC coupled with ESI-MS/MS: application to a bioequivalence study.Sci Pharm. 2010 Jul-Sep;78(3):411-22. doi: 10.3797/scipharm.1002-07. Epub 2010 Jun 4. Sci Pharm. 2010. PMID: 21179354 Free PMC article.
Cited by
-
LC-MS/MS determination and pharmacokinetic study of bergenin, the main bioactive component of Bergenia purpurascens after oral administration in rats.J Pharm Anal. 2013 Aug;3(4):229-234. doi: 10.1016/j.jpha.2013.01.005. Epub 2013 Jan 24. J Pharm Anal. 2013. PMID: 29403822 Free PMC article.
-
Bioanalytical method development and validation of milnacipran in rat plasma by LC-MS/MS detection and its application to a pharmacokinetic study.J Pharm Anal. 2013 Dec;3(6):481-488. doi: 10.1016/j.jpha.2013.03.009. Epub 2013 Apr 29. J Pharm Anal. 2013. PMID: 29403859 Free PMC article.
-
Method development and validation of Guanfacine in rat plasma by liquid chromatography-tandem mass spectrometry: Application to a pharmacokinetic study.J Pharm Anal. 2013 Dec;3(6):472-480. doi: 10.1016/j.jpha.2013.04.006. Epub 2013 Jun 27. J Pharm Anal. 2013. PMID: 29403858 Free PMC article.
-
A validated LC-MS/MS method for the determination of tolterodine and its metabolite in rat plasma and application to pharmacokinetic study.J Pharm Anal. 2013 Dec;3(6):489-499. doi: 10.1016/j.jpha.2013.04.005. Epub 2013 May 24. J Pharm Anal. 2013. PMID: 29403860 Free PMC article.
-
Quantitation of bivalirudin, a novel anticoagulant peptide, in human plasma by LC-MS/MS: Method development, validation and application to pharmacokinetics.J Pharm Anal. 2013 Feb;3(1):1-8. doi: 10.1016/j.jpha.2012.10.006. Epub 2012 Nov 7. J Pharm Anal. 2013. PMID: 29403790 Free PMC article.
References
-
- Zheng J., Rustum A.M. Rapid separation of desloratadine and related compounds in solidpharmaceutical formulation using gradient ion-pair chromatography. J. Pharm. Biomed. Anal. 2010;51(1):146–152. - PubMed
-
- Ramanathan R., Reyderman L., Kulmatycki K. Disposition of loratadine in healthy volunteers. Xenobiotica. 2007;37(7):753–769. - PubMed
-
- Ramanathan R., Alvarez N., Su A.D. Metabolism and excretion of loratadine in male and female mice, rats and monkeys. Xenobiotica. 2005;35(2):155–189. - PubMed
-
- Ghosal A., Yuan Y., Hapangama N. Identification of human UDP-glucuronosyltransferase enzyme(s) responsible for the glucuronidation of 3-hydroxydesloratadine. Biopharm. Drug Dispos. 2004;25(6):243–252. - PubMed
-
- Yeh G.C., Deng S.T., Lo C.Y. Pharmacokinetics and bioequivalence study of a generic desloratadine tablet formulation in healthy male volunteers. Arzneimittelforschung. 2004;54(3):166–170. - PubMed
LinkOut - more resources
Full Text Sources

