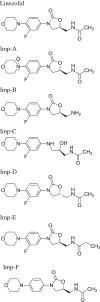
A validated stability-indicating LC method for the separation of enantiomer and potential impurities of Linezolid using polar organic mode
- PMID: 29403753
- PMCID: PMC5760913
- DOI: 10.1016/j.jpha.2012.03.006
A validated stability-indicating LC method for the separation of enantiomer and potential impurities of Linezolid using polar organic mode
Abstract
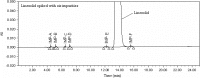
Although a number of methods are available for evaluating Linezolid and its possible impurities, a common method for separation if its potential impurities, degradants and enantiomer in a single method with good efficiency remain unavailable. With the objective of developing an advanced method with shorter runtimes, a simple, precise, accurate stability-indicating LC method was developed for the determination of purity of Linezolid drug substance and drug products in bulk samples and pharmaceutical dosage forms in the presence of its impurities and degradation products. This method is capable of separating all the related substances of Linezolid along with the chiral impurity. This method can also be used for the estimation of assay of Linezolid in drug substance as well as in drug product. The method was developed using Chiralpak IA (250 mm×4.6 mm, 5 μm) column. A mixture of acetonitrile, ethanol, n-butyl amine and trifluoro acetic acid in 96:4:0.10:0.16 (v/v/v/v) ratio was used as a mobile phase. The eluted compounds were monitored at 254 nm. Linezolid was subjected to the stress conditions of oxidative, acid, base, hydrolytic, thermal and photolytic degradation. The degradation products were well resolved from main peak and its impurities, proving the stability-indicating power of the method. The developed method was validated as per International Conference on Harmonization (ICH) guidelines with respect to specificity, limit of detection, limit of quantification, precision, linearity, accuracy, robustness and system suitability.
Keywords: HPLC; Linezolid; Polar organic mode; Stability-indicating; Validation.
Figures
References
-
- Gray E.Z., Charles W.F., Douglas K.H., Steven J.B., Michael R.B. development of the clinical candidates eperezolid and linezolid. Expert Opin. Investig. Drugs. 1997;6(2):151–158. - PubMed
-
- Barrett J.F. Linezolid pharmacia corp. Curr. Opin. Investig. Drugs. 2000;1(2):181–/187. - PubMed
-
- Borner K., Borner E., Lode H. Determination of linezolid in human serum and urine by high-performance liquid chromatography. Int. J. Antimicrob. Agents. 2001;18(3):253–258. - PubMed
-
- Peng G.W., Stryd R.P., Murata S. Determination of linezolid in plasma by reversed-phase high-performance liquid chromatography. J. Pharm. Biomed. Anal. 1999;20:65–73. - PubMed
LinkOut - more resources
Full Text Sources