Simultaneous determination of telmisartan and amlodipine in human plasma by LC-MS/MS and its application in a human pharmacokinetic study
- PMID: 29403761
- PMCID: PMC5760765
- DOI: 10.1016/j.jpha.2012.03.008
Simultaneous determination of telmisartan and amlodipine in human plasma by LC-MS/MS and its application in a human pharmacokinetic study
Abstract
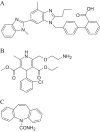
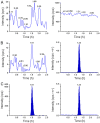
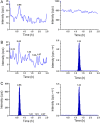
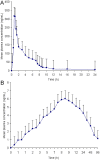
A rapid and sensitive liquid chromatography-tandem mass spectrometric (LC-MS/MS) assay method has been developed and fully validated for the simultaneous quantification of telmisartan and amlodipine in human plasma. Carbamazepine was used as an internal standard. Analytes and the internal standard were extracted from human plasma by solid-phase extraction technique using Waters Oasis® HLB 1 cm3 (30 mg) extraction cartridge. The reconstituted samples were chromatographed on a Hypurity advance C18 column (50 mm×4.6 mm, 5 μm) using a mixture of acetonitrile-5 mM ammonium acetate buffer (pH-4.0) (50:50, v/v) as the mobile phase at a flow rate of 0.8 mL/min. The calibration curve obtained was linear (r≥0.99) over the concentration range of 2.01-400.06 ng/mL for telmisartan and 0.05-10.01 ng/mL for amlodipine. Method validation was performed as per FDA guidelines and the results met the acceptance criteria. A run time of 2.5 min for each sample made it possible to analyze more than 400 human plasma samples per day. The proposed method was found to be applicable to clinical studies.
Keywords: Amlodipine; Human plasma; LC–MS/MS; Pharmacokinetics; Solid-phase extraction; Telmisartan.
Figures

Similar articles
-
Simultaneous determination of losartan, losartan acid and amlodipine in human plasma by LC-MS/MS and its application to a human pharmacokinetic study.Pharm Methods. 2012 Jan;3(1):18-25. doi: 10.4103/2229-4708.97711. Pharm Methods. 2012. PMID: 23781473 Free PMC article.
-
High performance liquid chromatography mass spectrometric method for the simultaneous quantification of pravastatin and aspirin in human plasma: Pharmacokinetic application.J Pharm Anal. 2012 Jun;2(3):206-213. doi: 10.1016/j.jpha.2012.01.001. Epub 2012 Feb 4. J Pharm Anal. 2012. PMID: 29403744 Free PMC article.
-
Simultaneous quantification of lidocaine and prilocaine in human plasma by LC-MS/MS and its application in a human pharmacokinetic study.Pract Lab Med. 2019 Jul 30;17:e00129. doi: 10.1016/j.plabm.2019.e00129. eCollection 2019 Nov. Pract Lab Med. 2019. PMID: 31414038 Free PMC article.
-
Simultaneous determination of pioglitazone and candesartan in human plasma by LC-MS/MS and its application to a human pharmacokinetic study.J Pharm Anal. 2012 Jun;2(3):167-173. doi: 10.1016/j.jpha.2012.01.002. Epub 2012 Jan 28. J Pharm Anal. 2012. PMID: 29403738 Free PMC article.
-
Simultaneous determination of atorvastatin, metformin and glimepiride in human plasma by LC-MS/MS and its application to a human pharmacokinetic study.J Pharm Anal. 2013 Feb;3(1):9-19. doi: 10.1016/j.jpha.2012.09.002. Epub 2012 Oct 3. J Pharm Anal. 2013. PMID: 29403791 Free PMC article.
Cited by
-
Simultaneous quantification of prodrug oseltamivir and its metabolite oseltamivir carboxylate in human plasma by LC-MS/MS to support a bioequivalence study.J Pharm Anal. 2013 Jun;3(3):149-160. doi: 10.1016/j.jpha.2012.11.004. Epub 2012 Dec 6. J Pharm Anal. 2013. PMID: 29403810 Free PMC article.
-
Development and validation of a high throughput LC-MS/MS method for simultaneous quantitation of pioglitazone and telmisartan in rat plasma and its application to a pharmacokinetic study.J Pharm Anal. 2017 Dec;7(6):381-387. doi: 10.1016/j.jpha.2017.05.004. Epub 2017 May 19. J Pharm Anal. 2017. PMID: 29404063 Free PMC article.
-
Application of an LC-MS/MS method for the analysis of amlodipine, valsartan and hydrochlorothiazide in polypill for a bioequivalence study.J Pharm Anal. 2017 Oct;7(5):309-316. doi: 10.1016/j.jpha.2017.06.001. Epub 2017 Jun 4. J Pharm Anal. 2017. PMID: 29404054 Free PMC article.
-
Simultaneous determination of rosuvastatin and amlodipine in human plasma using tandem mass spectrometry: Application to disposition kinetics.J Adv Res. 2015 Nov;6(6):931-40. doi: 10.1016/j.jare.2014.08.010. Epub 2014 Sep 6. J Adv Res. 2015. PMID: 26644931 Free PMC article.
-
Design of Experimental Approach for Development of Rapid High Performance Liquid Chromatographic Process for Simultaneous Estimation of Metoprolol, Telmisartan, and Amlodipine from Formulation: Greenness and Whiteness Evaluation.Molecules. 2024 Feb 29;29(5):1087. doi: 10.3390/molecules29051087. Molecules. 2024. PMID: 38474605 Free PMC article.
References
-
- Ruth R.W., William J.C., John D.I. Nonpeptide angiotensin II receptor antagonists: the next generation in antihypertensive therapy. J. Med. Chem. 1996;39:625–656. - PubMed
-
- Pitt B., Konstam M.A. Overview of angiotensin II-receptor antagonists. Am. J. Cardiol. 1998;82:47S–49S. - PubMed
-
- Yusuf S., Teo K.K., Pogue J. Telmisartan, ramipril, or both in patients at high risk for vascular events. N. Engl. J. Med. 2008;358:1547–1559. - PubMed
-
- Abernethy DR. Pharmacokinetics and pharmacodynamics of amlodipine. Cardiology. 1992;80:31–36. - PubMed
-
- Kungys G., Naujoks H., Wanner C. Pharmacokinetics of amlodipine in hypertensive patients undergoing haemodialysis. Eur. J. Clin. Pharmacol. 2003;59:291–295. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

