Quantitation of bivalirudin, a novel anticoagulant peptide, in human plasma by LC-MS/MS: Method development, validation and application to pharmacokinetics
- PMID: 29403790
- PMCID: PMC5760933
- DOI: 10.1016/j.jpha.2012.10.006
Quantitation of bivalirudin, a novel anticoagulant peptide, in human plasma by LC-MS/MS: Method development, validation and application to pharmacokinetics
Abstract
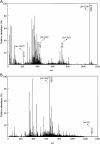
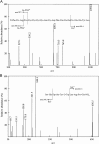
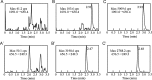
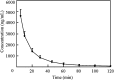
A rapid and sensitive method based on liquid chromatography-tandem mass spectrometry (LC-MS/MS) for the determination of a novel anticoagulant peptide bivalirudin in human plasma has been developed and validated. Plasma samples were precipitated protein with acetonitrile and re-extracted with dichloromethane, after which the analyte and triptorelin as an internal standard (IS) were separated on a 300SB-C18 column (150 mm×4.6 mm i.d., 5 μm particle size) using 0.1% formic acid:methanol (45:55, v/v) as mobile phase. The triple-quadrupole mass spectrometer, equipped with electrospray ionization (ESI) interface, was operated in the positive ion mode, and the multiple-reaction monitoring (MRM) transitions of bivalirudin and IS were at m/z 1091.0→650.4 and m/z 656.5→249.3, respectively. The lower limit of quantification (LLOQ) was 1 ng/mL for 100 μL plasma sample and the assay was linear over the concentration range 1-1000 ng/mL. The accuracy was within a range from -0.4% to 0.5% in terms of relative error (RE) and the intra- and inter-day precisions in terms of relative standard deviation (RSD) were ≤2.92 and ≤3.36, respectively. The method was successfully applied to a pharmacokinetic study involving intravenous administration of bivalirudin (0.5 mg/kg) to Chinese volunteers.
Keywords: Anticoagulant; Bivalirudin; Human plasma; LC–MS/MS; Pharmacokinetics.
Figures

Similar articles
-
Development and validation of a highly sensitive LC-MS/MS method for quantitation of bivalirudin in human plasma: application to a human pharmacokinetic study.Biomed Chromatogr. 2013 Dec;27(12):1788-93. doi: 10.1002/bmc.2998. Epub 2013 Jul 25. Biomed Chromatogr. 2013. PMID: 23893840 Clinical Trial.
-
Determination of thalidomide concentration in human plasma by liquid chromatography-tandem mass spectrometry.Exp Ther Med. 2013 Feb;5(2):626-630. doi: 10.3892/etm.2012.847. Epub 2012 Nov 30. Exp Ther Med. 2013. PMID: 23404219 Free PMC article.
-
Validated LC-MS/MS method for quantitative determination of rasagiline in human plasma and its application to a pharmacokinetic study.J Chromatogr B Analyt Technol Biomed Life Sci. 2008 Oct 1;873(2):203-8. doi: 10.1016/j.jchromb.2008.08.024. Epub 2008 Sep 5. J Chromatogr B Analyt Technol Biomed Life Sci. 2008. PMID: 18799367
-
Determination of songorine in rat plasma by UPLC-MS/MS: Assay development and application to pharmacokinetic study.J Chromatogr B Analyt Technol Biomed Life Sci. 2015 Oct 1;1002:234-8. doi: 10.1016/j.jchromb.2015.08.037. Epub 2015 Aug 28. J Chromatogr B Analyt Technol Biomed Life Sci. 2015. PMID: 26342165
-
The correlation between the level of 3-hydroxypropyl mercapturic acid, CYP2B6 polymorphisms, and hematuria occurrences after cyclophosphamide administration and its bioanalytical methods: A systematic review.Heliyon. 2021 Oct 4;7(10):e08126. doi: 10.1016/j.heliyon.2021.e08126. eCollection 2021 Oct. Heliyon. 2021. PMID: 34746455 Free PMC article. Review.
Cited by
-
Development of an LC-MS/MS method for determination of 2-oxo-clopidogrel in human plasma.J Pharm Anal. 2015 Feb;5(1):12-17. doi: 10.1016/j.jpha.2014.07.004. Epub 2014 Jul 17. J Pharm Anal. 2015. PMID: 29403910 Free PMC article.
-
The Pharmacokinetics in Mice and Cell Uptake of Thymus Immunosuppressive Pentapeptide Using LC-MS/MS Analysis.Molecules. 2022 Jul 1;27(13):4256. doi: 10.3390/molecules27134256. Molecules. 2022. PMID: 35807500 Free PMC article.
-
Pharmacokinetic study of inosiplex tablets in healthy Chinese volunteers by hyphenated HPLC and tandem MS techniques.J Pharm Anal. 2013 Dec;3(6):387-393. doi: 10.1016/j.jpha.2013.03.001. Epub 2013 Mar 20. J Pharm Anal. 2013. PMID: 29403844 Free PMC article.
References
-
- Warkentin T.E. Bivalent direct thrombin inhibitors: hirudin and bivalirudin. Best Pract. Res. Clin. Haematol. 2004;17:105–125. - PubMed
-
- Rha S.W., Kuchulakanti P.K., Pakala R. Bivalirudin versus heparin as an antithrombotic agent in patients treated with a sirolimus-eluting stent. Am. J. Cardiol. 2004;94:1047–1050. - PubMed
-
- Gladwell T.D. Bivalirudin: a direct thrombin inhibitor. Clin. Ther. 2002;24:38–58. - PubMed
-
- Gurm H.S., Rajagopal V., Fathi R. Effectiveness and safety of bivalirudin during percutaneous coronary intervention in a single medical center. Am. J. Cardiol. 2005;95:716–721. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

