Simultaneous determination of atorvastatin, metformin and glimepiride in human plasma by LC-MS/MS and its application to a human pharmacokinetic study
- PMID: 29403791
- PMCID: PMC5760920
- DOI: 10.1016/j.jpha.2012.09.002
Simultaneous determination of atorvastatin, metformin and glimepiride in human plasma by LC-MS/MS and its application to a human pharmacokinetic study
Abstract
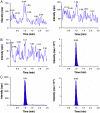
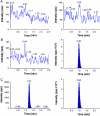
A simple, rapid and sensitive liquid chromatography-tandem mass spectrometric (LC-MS/MS) assay method has been developed and fully validated for the simultaneous quantification of atorvastatin, metformin and glimepiride in human plasma. Carbamazepine was used as internal standard (IS). The analytes were extracted from 200 μL aliquots of human plasma via protein precipitation using acetonitrile. The reconstituted samples were chromatographed on a Alltima HP C18 column by using a 60:40 (v/v) mixture of acetonitrile and 10 mM ammonium acetate (pH 3.0) as the mobile phase at a flow rate of 1.1 mL/min. The calibration curves obtained were linear (r2 ≥0.99) over the concentration range of 0.50-150.03 ng/mL for atorvastatin, 12.14-1207.50 ng/mL for metformin and 4.98-494.29 ng/mL for glimepiride. The API-4000 LC-MS/MS in multiple reaction monitoring (MRM) mode was used for detection. The results of the intra- and inter-day precision and accuracy studies were well within the acceptable limits. All the analytes were found to be stable in a battery of stability studies. The method is precise and sensitive enough for its intended purpose. A run time of 2.5 min for each sample made it possible to analyze more than 300 plasma samples per day. The developed assay method was successfully applied to a pharmacokinetic study in human male volunteers.
Keywords: Atorvastatin; Glimepiride; Human plasma; LC–MS/MS; Metformin; Pharmacokinetics.
Figures




Similar articles
-
Simultaneous determination of sitagliptin and simvastatin in human plasma by LC-MS/MS and its application to a human pharmacokinetic study.Biomed Chromatogr. 2013 Jan;27(1):80-7. doi: 10.1002/bmc.2751. Epub 2012 Apr 30. Biomed Chromatogr. 2013. PMID: 22544712
-
Simultaneous determination of pioglitazone and candesartan in human plasma by LC-MS/MS and its application to a human pharmacokinetic study.J Pharm Anal. 2012 Jun;2(3):167-173. doi: 10.1016/j.jpha.2012.01.002. Epub 2012 Jan 28. J Pharm Anal. 2012. PMID: 29403738 Free PMC article.
-
Simultaneous determination of telmisartan and amlodipine in human plasma by LC-MS/MS and its application in a human pharmacokinetic study.J Pharm Anal. 2012 Oct;2(5):319-326. doi: 10.1016/j.jpha.2012.03.008. Epub 2012 Apr 4. J Pharm Anal. 2012. PMID: 29403761 Free PMC article.
-
Simultaneous Determination of Atorvastatin and Aspirin in Human Plasma by LC-MS/MS: Its Pharmacokinetic Application.Sci Pharm. 2012 Oct-Dec;80(4):923-40. doi: 10.3797/scipharm.1206-12. Epub 2012 Aug 6. Sci Pharm. 2012. PMID: 23264940 Free PMC article.
-
Liquid chromatography-tandem mass spectrometric assay for the determination of tetrabenazine and its active metabolites in human plasma: a pharmacokinetic study.Biomed Chromatogr. 2013 Jun;27(6):792-801. doi: 10.1002/bmc.2862. Epub 2013 Jan 21. Biomed Chromatogr. 2013. PMID: 23339053
Cited by
-
Stability Indicating RP-HPLC Method for the Simultaneous Determination of Atorvastatin Calcium, Metformin Hydrochloride, and Glimepiride in Bulk and Combined Tablet Dosage Form.Int Sch Res Notices. 2014 Oct 29;2014:754695. doi: 10.1155/2014/754695. eCollection 2014. Int Sch Res Notices. 2014. PMID: 27433531 Free PMC article.
-
An integrated Taguchi and response surface methodological approach for the optimization of an HPLC method to determine glimepiride in a supersaturatable self-nanoemulsifying formulation.Saudi Pharm J. 2016 Jan;24(1):92-103. doi: 10.1016/j.jsps.2015.03.004. Epub 2015 Mar 23. Saudi Pharm J. 2016. PMID: 26903773 Free PMC article.
-
The Clinical Pharmacokinetics and Pharmacodynamics of Glimepiride-A Systematic Review and Meta-Analysis.Pharmaceuticals (Basel). 2025 Jan 17;18(1):122. doi: 10.3390/ph18010122. Pharmaceuticals (Basel). 2025. PMID: 39861183 Free PMC article.
-
Development and Validation of Liquid Chromatography Method for Determination of Glimepiride in Presence of (Vimto®) Soft Drinks in Rats: Application to Pharmacokinetics Studies.J Pharm Bioallied Sci. 2019 Jan-Mar;11(1):49-59. doi: 10.4103/jpbs.JPBS_200_18. J Pharm Bioallied Sci. 2019. PMID: 30906140 Free PMC article.
-
Pharmacokinetic study of inosiplex tablets in healthy Chinese volunteers by hyphenated HPLC and tandem MS techniques.J Pharm Anal. 2013 Dec;3(6):387-393. doi: 10.1016/j.jpha.2013.03.001. Epub 2013 Mar 20. J Pharm Anal. 2013. PMID: 29403844 Free PMC article.
References
-
- American Diabetes Association Standards of medical care for patients with diabetes mellitus (Position Statement) Diabetes Care. 2003;26(Suppl. 1):S33–S50. - PubMed
-
- Bakker-Arkema R.G., Davidson M.H., Goldstein R.J. Efficacy and safety of a new HMG-CoA reductase inhibitor, atorvastatin, in patients with hypertriglyceridemia. J. Am. Med. Assoc. 1996;275:128–133. - PubMed
-
- Nawrocki J.W., Weiss S.R., Davidson M.H. Reduction of LDL cholesterol by 25% to 60% in patients with primary hypercholesterolemia by atorvastatin, a new HMG-CoA reductase inhibitor. Arterioscler. Thromb. Vasc. Biol. 1995;15:678–682. - PubMed
-
- Krentz A.J., Bailey C.J. Oral antidiabetic agents: current role in type 2 diabetes mellitus. Drugs. 2005;65:385–411. - PubMed
-
- DeFronzo R.A., Barzilai N., Simonson D.C. Mechanism of metformin action in obese and lean noninsulin-dependent diabetic subjects. J. Clin. Endocrinol. Metab. 1991;73:1294–1301. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

