Liquid chromatography tandem mass spectrometry method for the estimation of lamotrigine in human plasma: Application to a pharmacokinetic study
- PMID: 29403800
- PMCID: PMC5760919
- DOI: 10.1016/j.jpha.2012.09.001
Liquid chromatography tandem mass spectrometry method for the estimation of lamotrigine in human plasma: Application to a pharmacokinetic study
Abstract
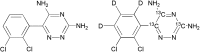
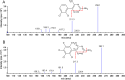
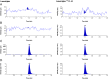
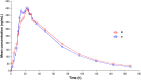
A reliable, selective and sensitive liquid chromatography tandem mass spectrometry method was developed and validated for the quantification of lamotrigine in human plasma using lamotrigine-13C3, d3 as an internal standard. Analyte and internal standard were extracted from human plasma by solid-phase extraction and detected in positive ion mode by tandem mass spectrometry with electrospray ionization (ESI) interface. Chromatographic separation was performed on a Chromolith® SpeedROD; RP-18e column (50-4.6 mm i.d.) using acetonitrile: 5±0.1 mM ammonium formate solution (90:10, v/v) as the mobile phase at a flow rate of 0.500 mL/min. The calibration curves were linear over the range of 5.02-1226.47 ng/mL with the lower limit of quantitation validated at 5.02 ng/mL. The analytes were found stable in human plasma through three freeze (-20 °C)-thaw (ice-cold water bath) cycles and under storage on bench-top in ice-cold water bath for at least 6.8 h, and also in the mobile phase at 10 °C for at least 57 h. The method has shown good reproducibility, as the intra- and inter-day precisions were within 3.0%, while the accuracies were within ±6.0% of nominal values. The validated LC-MS/MS method was applied for the evaluation of pharmacokinetic and bioequivalence parameters of lamotrigine after an oral administration of 50 mg lamotrigine tablet to thirty-two healthy adult male volunteers.
Keywords: Lamotrigine; Liquid chromatography/tandem mass spectrometry; Pharmacokinetic study; Solid phase extraction.
Figures

Similar articles
-
Liquid chromatography/electrospray tandem mass spectrometry method for the determination of cefuroxime in human plasma: application to a pharmacokinetic study.J Chromatogr B Analyt Technol Biomed Life Sci. 2010 Feb 1;878(3-4):428-34. doi: 10.1016/j.jchromb.2009.12.025. Epub 2010 Jan 4. J Chromatogr B Analyt Technol Biomed Life Sci. 2010. PMID: 20060790 Clinical Trial.
-
Development and Validation of a LC-MS/MS Method for the Simultaneous Estimation of Amlodipine and Valsartan in Human Plasma: Application to a Bioequivalence Study.Sci Pharm. 2014 Mar 26;82(3):585-600. doi: 10.3797/scipharm.1402-11. Print 2014 Jul-Sep. Sci Pharm. 2014. PMID: 25853070 Free PMC article.
-
Determination of tegaserod by LC-ESI-MS/MS and its application to a pharmacokinetic study in healthy Chinese volunteers.J Chromatogr B Analyt Technol Biomed Life Sci. 2008 Jan 1;861(1):151-7. doi: 10.1016/j.jchromb.2007.11.011. Epub 2007 Nov 19. J Chromatogr B Analyt Technol Biomed Life Sci. 2008. PMID: 18069077 Clinical Trial.
-
Rapid and sensitive LC-MS/MS method for quantification of lamotrigine in human plasma: application to a human pharmacokinetic study.Biomed Chromatogr. 2012 Apr;26(4):491-6. doi: 10.1002/bmc.1692. Epub 2011 Sep 8. Biomed Chromatogr. 2012. PMID: 21905057
-
Quantification of desloratadine in human plasma by LC-ESI-MS/MS and application to a pharmacokinetic study.J Pharm Anal. 2012 Jun;2(3):180-187. doi: 10.1016/j.jpha.2012.01.008. Epub 2012 Jan 31. J Pharm Anal. 2012. PMID: 29403740 Free PMC article.
Cited by
-
Determination of Ethinyl Estradiol and Levonorgestrel in Human Plasma with Prednisone as Internal Standard Using Ultra-performance Liquid Chromatography-Tandem Mass Spectrometry.J Pharm Bioallied Sci. 2019 Jul-Sep;11(3):254-261. doi: 10.4103/jpbs.JPBS_68_19. J Pharm Bioallied Sci. 2019. PMID: 31555032 Free PMC article.
-
Development and Validation of Doxorubicin Hydrochloride and Doxorubicinol Quantification Method in Dried Blood Spot by Liquid Chromatography-Tandem Mass Spectrometry.J Pharm Bioallied Sci. 2020 Oct-Dec;12(4):406-412. doi: 10.4103/jpbs.JPBS_167_20. Epub 2020 Oct 8. J Pharm Bioallied Sci. 2020. PMID: 33679086 Free PMC article.
-
Development of magnetic molecularly imprinted polymers with double templates for the rapid and selective determination of carbamazepine and lamotrigine in serum.RSC Adv. 2022 Mar 30;12(16):10051-10061. doi: 10.1039/d1ra09306a. eCollection 2022 Mar 25. RSC Adv. 2022. PMID: 35424933 Free PMC article.
-
Simultaneous determination of asenapine and valproic acid in human plasma using LC-MS/MS: Application of the method to support pharmacokinetic study.J Pharm Anal. 2013 Dec;3(6):394-401. doi: 10.1016/j.jpha.2013.04.008. Epub 2013 May 23. J Pharm Anal. 2013. PMID: 29403845 Free PMC article.
-
Method Development and Validation for Measuring O6-Methylguanine in Dried Blood Spot Using Ultra High-Performance Liquid Chromatography Tandem Mass Spectrometry.Drug Des Devel Ther. 2021 Mar 3;15:963-971. doi: 10.2147/DDDT.S283775. eCollection 2021. Drug Des Devel Ther. 2021. PMID: 33692614 Free PMC article.
References
-
- Miller A.A., Wheatley P., Sawyer D.A. Pharmacological studies on lamotrigine, a novel potential antiepileptic drug: I. Anticonvulsant profile in mice and rats. Epilepsia. 1986;27:483–489. - PubMed
-
- Cohen A.F., Land G.S., Breimer D.D. Lamotrigine, a new anticonvulsant: pharmacokinetics in normal humans. Clin. Pharmacol. Ther. 1987;42:535–541. - PubMed
-
- Biddlecombe R.A., Dean K.L., Smith C.D. Validation of a radioimmunoassay for the determination of human plasma concentrations of lamotrigine. J. Pharm. Biomed. Anal. 1990;8:691–694. - PubMed
-
- Sinz M.W., Remmel R.P. Analysis of lamotrigine and lamotrigine 2-N-glucuronide in guinea pig blood and urine by reserved-phase ion-pairing liquid chromatography. J. Chromatogr. 1991;571:217–230. - PubMed
-
- Yamashita S., Furuno K., Kawasaki H. Simple and rapid analysis of lamotrigine, a novel antiepileptic, in human serum by high-performance liquid chromatography using a solid-phase extraction technique. J. Chromatogr. B. 1995;670:354–357. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

